Introduction
Constant deployment of antimicrobial medications in treating diseases has prompted the emergence of resistance among different pathogens (S. Singh et al., 2010). Nanodrugs where nanoparticles, have promising applications in drug delivery systems and diagnostics for the treatment of different diseases (Marcato & Durán, 2008; R. Singh & Nalwa, 2011). Liposomes are appropriate as vehicles for transporting antimicrobials since they generally provide a safe release of drug, reduced toxicity and protection of the encapsulated drug from becoming ineffective (Omri et al., 2002).
Upon direct fusion, the encapsulated or entrapped antimicrobial drug inside the liposomes can be discharged to the bacterial cell membrane or inside the microorganism. The exceptional structure of liposomes, a lipid layer encompassing an aqueous cavity, empowers them to convey both hydrophobic and hydrophilic drugs, while inhibiting any chemical modification (Zhang et al., 2010). Hydrophilic drugs are entrapped inside the aqueous core while hydrophilic drugs are incorporated in the lipid bilayers of the liposomes (Ding et al., 2006). Various clinical formulations of liposomes for treating various diseases have been made (Immordino et al., 2006). The effect of certain antimicrobials against different infectious microbes is increased by entrapping inside the liposomes (Fenske et al., 2008). New approaches for novel therapeutic applications are strengthened by consolidating the properties of liposomal nanocarriers and nanoparticles. The impact of nanoparticles incorporation on the lipid bilayers of liposome was also observed (Rivera Gil et al., 2010). Nanoparticles are incorporated inside the liposomal vesicles for a number of applications, providing extraordinary advances in drug delivery systems (Al-jamal & Kostarelos, 2007).
Silver nanoparticle’s efficacy against many bacteria was explored by several researchers and their successful potential in opposition to a wide variety of microorganisms was demonstrated. At the start of the twentieth century, Mukherjee and co-workers reported the first production of silver nanoparticles by means of a fungus-assisted method (Mukherjee et al., 2001). For the synthesis of silver nanoparticles of the diameter ranging from 25±12 nm, the fungus Verticillium was utilized. Husseiny with his co-worker in 2015 synthesized Ag nanoparticles from Fusarium oxysporum and evaluated their antimicrobial activity against pathogenic bacteria through Staphlococcus aureus, and Escherichia coli (Husseiny et al., 2015).
In one of the previous study the combined effectiveness of biologically synthesized silver nanoparticles and antibiotics of different types against bacteria that were multidrug resistant was also reported (Naqvi et al., 2013). Schumacher and Margalit (1997), employed lipid thin film hydration method for the preparation of liposome encapsulated ampicillin demonstrated polymyxin B encapsulated liposomes as another effective example of antibacterial liposomal drug delivery systems.
Hădărugă et al. (2011) synthesized stable liposomes containing cobalt ferrite nanoparticles using an ultrasonication method. The development of nanoliposomes containing silver nanoparticles as a potential antimicrobial tool against microbial resistance was reported by Eid and Azzazy (2014). Dextrose-capped spherical silver nanoparticles were prepared and incorporated into nanoliposomes, resulting in the silver nanoparticles incorporating nanoliposomes of a size ranging from 25-80 nm by using reverse phase evaporation method. Nanoparticle/liposome interactions are thus playing a vital role in the research development of nanomedicine. Liposomes serve as model systems by consolidating the properties of silver nanoparticles and lipid vesicles, resulting in novel nanomedical applications. Nanoparticles are thus used while preparing liposomes in order to control the membrane properties of vesicles. For the photo-induced release of drugs, fluorescent silver nanoparticles were embedded in the bilayers of liposomes by Li et al. (2014). Thus, in the current study, mycosynthesized silver nanoparticles will be incorporated in liposomes to find their effectiveness in the drug delivery system.
Materials and methods
Mycosynthesis of silver nanoparticles
For microbial synthesis of extracellular silver nanoparticles, the fungal strain Aspergillus fumigatus BTCB01 was obtained from the Culture Bank BTCB of Lahore College for Women University, Department of Biotechnology (Shahzad et al., 2019).
Biomass preparation
Biomass for this purpose was prepared in the minimal media with neutral pH comprising glucose (0.75%), MgSO4•7H2O (0.30 mM), KH2PO4 (38.6 mM), K2HPO4 (8.63 mM), (NH4)2SO4 (5.68 mM), and yeast extract (0.045%). Media was inoculated with a loop full of spores and incubated at 25°C with a continuous shaking at 120 rpm for 72 hours (Madakka et al., 2018). Biomass was filtered using Whatman filter paper (grade A) no. 1 (Ahlstrom, Spain) and was resuspended in double distilled water (150 mL) in a 250 ml Erlenmeyer flask after rinsing it three times, and later it was incubated at 25°C with continuous shaking (120 rpm) for 72 hours.
Addition of salt in nanoparticle synthesis
After 72 hours of shaking, the biomass was again filtered using filter paper and the remaining cell-free extract was obtained. Silver nitrate (1 mM) was added to 100 mL of distilled water in 1:1 ratio. The flasks were incubated at 25°C at 120 rpm until the color change occurred, indicating the formation of silver nanoparticles (Sadowski et al., 2008).
Preparation of liposomes incorporating silver nanoparticles and drug encapsulation
Liposomes were prepared using the thin film hydration method as described previously (Fritze et al., 2006). For this purpose, 20 mg of PC (phosphatidylcholine) and 13.1 mg of CH (cholesterol) (3:1) were dissolved in the organic solvent methanol:chloroform (10 ml) (2:1, v/v) in a round bottom flask. Further, the evaporation of organic solvent was carried out using a rotary evaporator under high vacuum in a 40°C water bath and rotated at 180 rpm until the thin dry film of lipid was deposited on the wall of the flask. The swelling solution comprising 5 ml of (PBS) phosphate buffer saline (pH 7.6) was added to a previously prepared thin dry film and the flask containing the mixture solution was then again rotated for 30 minutes. Formed liposomes were (MLVs) multi-lamellar vesciles. For downsizing, MLVs were subjected to sonication for 30 minutes by placing them in a bath sonicator to obtain the (SUVs) small unilamellar vesciles. For meropenem, the chemical structure shown in Fig.1) encapsulated liposomes, the thin and dry lipid film was hydrated by adding 5 ml of antibiotic solution (32 mg of meropenem dissolved in 0.9% NaCl solution) as reported by (Gubernator et al., 2007). Then, after the incorporation of the drug in the liposomal solution Silver-nanoparticles (AgNPs) were added (5 ml) and hydrated by PBS in the mixture, according to Bernardi et al. (2012).
Figure 1. Chemical structure of Meropenem (Waheed et al., 2016).
Characterization of liposomes
Each liposomal formulation was characterized by measuring the mean particle size diameter, its zeta potential and polydispersity index immediately after the formation of the liposomal formulation at 25°C using the zeta sizer Nano ZS (Karn et al., 2013).
Drug quantification by HPLC
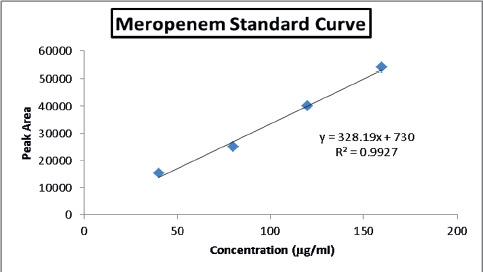
To quantify meropenem in the liposomes, reverse-phase HPLC was performed (Yng, 2005). An octadecylsilane (10 µm) column with ambient temperature was used for this purpose. The mobile phase comprises of 70% acetonitrile and 80% (KH2PO4) di-hydrogen potassium phosphate buffer (0.03M, pH 3). The liposomal samples (50 µl) were dissolved in organic solvent methanol (950 µl), with a flow rate of 1 ml/min in the column. The calibration of HPLC was done with the standard solutions with concentrations ranging from 40 to 160 µg/ml at 235 nm.
Encapsulation efficiency of liposomes
Encapsulation efficiency of liposomal formulations was calculated as reported in a previously published paper (Schumacher & Margalit, 1997; Yng, 2005). HPLC was used to assay the amount of meropenem in the pre-centrifuged sample, the resuspended pellet and the collected supernatant.
In vitro drug release study
A dialysis method was used for the in vitro meropenem release study of liposome dispersion as reported by Panwar et al. (2010). Dialysis bags (12,000-14,000 MW cut off; Sigma-Aldrich) were soaked prior to use in distilled water at room temperature for 12 h for the sake of removal of the preservative. Each dialysis bag contained 5 ml of free meropenem as control, 5 ml of drug-encapsulated liposome and 5 ml of nanoparticle incorporated drug liposome, respectively. For the drug release, each dialysis bag was separately immersed in a conical flask containing 100 ml of (PBS) phosphate buffer saline (pH 7.4) and stirred through magnetic stirrer at 100 rpm at 37 °C. Throughout the experiment, 1 ml of the aliquot at different time intervals was removed from the receptor chamber and replaced with an equivalent volume of fresh PBS. The release run continued for 24 hrs and after 1/2 hr, 1 hr, 3 hr, 5 hr, 8 hr, 24 hr the aliquots were taken and analyzed through HPLC.
In vitro antimicrobial assay against MDR Salmonella typhi BTCB170
The antibacterial activity was checked through the disk diffusion method (Choi et al., 2011). The multi-drug-resistant pathogen Salmonella typhi BTCB170 was used. For this purpose, S. typhi inoculum was prepared according to 0.5 McFarland standard. Nutrient agar plates were prepared and inoculated with the S. typhi inoculum by swabbing vertically and horizontally. Each disk was loaded with 20 µl of the prepared formulations and placed on the agar plates and incubated at 37°C for 24 hrs overnight. Inhibition zones were measured after 24 hours of incubation in millimeters.
Statistical Analysis
One-way ANOVA was used to find the significance (p ≤ 0.05) between means of different treatments. SPSS program version 16 was used for the analysis. Each experiment was repeated thrice.
Results and discussion
Mycosynthesis of silver nanoparticles
A colony of fungal strain Aspergillus fumigatus BTCB 01 on potato dextrose agar was obtained, which is shown in Figure 2.
Figure 2. Appearance of Aspergillus Fumigatus BTCB01; colony on potato dextrose agar medium after 7 days of incubation at 30°C.
The initial indication of the prepared silver nanoparticles was the change in color of the reaction mixture as shown in Figure 3. Further confirmation of AgNP’s presence was confirmed by the appearance of excitation peaks in the range of 300-376 nm in the UV-visible spectrum (data not shown).
Figure 3. Mycosynthesized AgNPs after mixing cell filtrate (72 h) of Aspergillus fumigatus BTCB01 with silver Figure nitrate (1 mM).

In the present study, the extracellular biosynthesis of fungal nanoparticles was carried out using the strain, Aspergillus fumigatus BTCB01. The fungal silver nanoparticles showed a polydispersity value of 0.4 due to no particle aggregations (Table 1). It is well known that nanoparticles having a PDI of less than 0.7 are stable, and no aggregation occurs, while the greater the zeta potential, the more stable the particles are. Many studies have shown the successful synthesis of silver nanoparticles of varying size and characteristics from different fungal species such as Trichoderma harzianum (Bawaskar et al., 2010; Shelar & Chavan, 2015), Aspergillus flavus (Jain et al., 2011) and Metarhizium anisopliae (Amerasan et al., 2015).
Table 1. Zeta analysis of different liposomal formulations.
| Experimental Groups | Liposomal Formulations | z-average diameter (nm) | Zeta Potential (mV) | Polydispersity Index |
|---|---|---|---|---|
| Group A | Liposomes Drug | 318.7 | -45.8 | 0.3 |
| Group B | encapsulated liposomes | 237.4 | -41.3 | 0.3 |
| Group C | Silver nanoparticle incorporated liposomes | 320.3 | -42.5 | 0.4 |
| Group D | Silver nanoparticle incorporated drug liposomes | 215.3 | -39.6 | 0.3 |
Synthesis and characterization of liposomes
Table 1 summarizes the z-average diameters in nm, zeta potential values and polydispersity index of all the liposomal formulations i.e. A (liposomes), B (drug-encapsulated liposomes), C (silver nanoparticles incorporating liposomes) and D (silver nanoparticles incorporating drug liposomes). Control (Formulation A) comprising of only liposomes had the z-average diameter of 318.7 nm, zeta potential of -45.8 mV and polydispersity of 0.3, indicating stable particles. Whereas, Formulation B, comprising drug-encapsulated liposomes, showed a z-average diameter of 237.4 nm with a zeta potential of -41.3 mV and polydispersity index value of 0.3, indicating the homogeneous stable liposomes. Formulation C (silver nanoparticle incorporated liposomes) exhibited greater particle stability with a z-average diameter of 320.3 nm and a zeta potential value of -42.5 mV, showing polydispersity of 0.4. Similarly, the formulation D (silver nanoparticles incorporating drug liposomes) had a z-average diameter of 215.3 nm and a zeta potential value of -39.6 mV with 0.3 polydispersity index, indicating no aggregations of liposomes. For the characterization of the present liposomal formulations, the mean particle diameters, zeta potential values and polydispersity index were determined by using zeta sizer Nano ZS. The samples were diluted in water and all the measurements were made at 25°C. This was in close agreement with (Hue et al., 2015) who characterized the liposomes size as 167.8± 3.6 nm while having negative zeta potential value of −27.5 ± 3.5 mV. A very similar study conducted previously also revealed the mean diameter of 278.46 nm and zeta potential of -18.3 mV of the silver-nanoparticle incorporated liposomes. Those results confirmed that obtained silver-nanoparticle incorporated liposomes had a mean diameter between 321 and 373 nm, with polydispersity index close to 0.2 and a negative zeta potential around -40 mV, indicating greater stability to the AgNPs (Espinoza et al., 2020).
Similarly, in another study using Aspergillus fumigatus and under optimized parameters, extracellular synthesis of AgNPs (yellow colored) was achieved within 20 seconds having 0.681 nm size at 400 nm with negative zeta potential of -23.4 mV, whereas in another previous study, extracellular brown-colored spherical AgNPs was also reported from Aspergillus flavus, synthesized within 72 hrs having an average size of 8.92 nm at 420 nm.
Drug quantification
In the present study, a reverse phase HPLC assay was performed for the quantification of meropenem in the liposomal formulations. By plotting the calibration plot of peak area versus meropenem standard concentration, all the four standard solutions of meropenem at the concentration of 40, 80, 120 and 160 µg/ml showed a good linear standard concentration curve having r2 = 0.99. Both the pure meropenem standard solutions and the liposomal samples of drug-encapsulated liposomes and silver nanoparticles incorporated liposomes before and after centrifugation showed a retention time of 3.3 minutes (Figure 4).
Figure 4. Standard curve for meropenem shows the retention time of 3.3 minutes.
Encapsulation efficiency of liposomes
The preparation of various liposomal formulations involving meropenem encapsulation as well as AgNPs incorporation in the liposomes using the thin film hydration method resulted in the development of stable liposomal formulations. Overall, the encapsulation efficiency of drug-encapsulated liposomes was greater than AgNPs incorporated drug liposomes. It was observed that drug encapsulated liposomes depicted 94% encapsulation efficiency (direct method) whereas 86% from AgNPs incorporated drug liposomes (Table 2). In one of the previously conducted studies, the encapsulation efficiency of silver nanoparticle incorporated liposomes was between 51.81 to 58.83%, which was less than drug-encapsulated liposomes (Espinoza et al., 2020). Whereas, 95% (indirect method) encapsulation efficiency was observed in drug encapsulated liposomes and 89% for encapsulation efficiency of AgNPs incorporated drug liposomes (Table 2). Thus, meropenem was found to be encapsulated more effectively in the drug-encapsulated liposomes as compared to AgNPs incorporated drug liposomes. AgNPs have strong interactions with the inner hydrophobic region of the liposomes. Changes in encapsulation efficiency might be due to the interaction of AgNPs with the hydrophobic part of liposomes, affecting the liposomal membrane fluidity, thereby effecting the drug release (Bothun, 2008).
Table 2. The percentage encapsulation efficiencies of liposomal formulations prepared.
| Experimental groups | Pre-centrifugationSample (μg) | After centrifugation Pellet sample(ug) | Supernatantsample (ug) | EE (IndirectMethod)(%) | EE (DirectMethod)(%) | Diameter of zone (mm)±SEM* |
|---|---|---|---|---|---|---|
| DrugEncapsulatedLiposomes | 25601 | 24111 | 1190 | 94 | 95 | 8.6±0.01e |
| Silver nanoparticlesdrugliposomes | 22321 | 22321 | 19321 | 86 | 89 | 8.4±0.01d |
In vitro drug release study
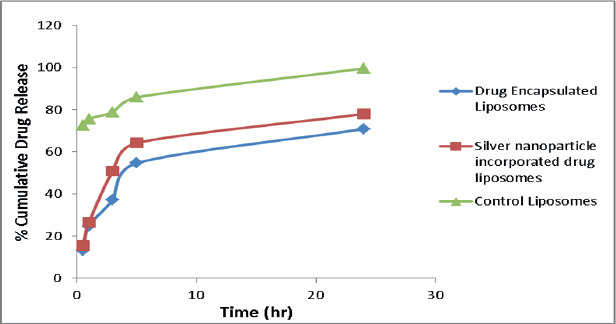
In vitro drug release study of both the drug and silver nanoparticle-incorporated liposomes showed sustained release of meropenem. The drug release rate from the liposomes increased gradually over 24 h and can be ranked in the order of drug-encapsulated liposomes > Silver nanoparticles incorporated drug liposomes > AgNPs (Figure 5). However, in a similar study, it was observed that the drug release from gold NPs encapsulated liposomes showed increased drug release and that is due to change in membrane fluidity because of the presence of metal nanoparticles (Patra et al., 2018).
Figure 5. In vitro drug release profile of all liposomal formulations.
In vitro antimicrobial activity
An In vitro study was done for the prepared liposomal formulations against the pathogenic bacteria Salmonella typhi BTCB170. Clear zones of inhibition around the loaded discs on the plates indicate the effectiveness of the formulation in inhibiting the growth of pathogens. In this respect, no zones of inhibition were observed by the plate-carrying disks loaded with control liposome and nanoparticle-incorporated liposomes. Whereas clear zones of inhibition were observed around the discs-loaded with silver nanoparticles (4 mm), drug-loaded liposomes (8.6 mm) and drug and NPs-loaded liposomes (8.4 mm) (Figure 6a-e). The antimicrobial activity decreases to 1fold with AgNPs and drug-encapsulated liposomes. Table 2 summarizes the diameter of zone of inhibition of drug in liposomal formulations. It was observed that formulations carrying AgNPs incorporating drug liposomes somehow reduced the antimicrobial activity by showing less zone of inhibition as compared to control samples. Reduction in inhibition zone might be related to the effect of AgNPs on the size and stability of the liposomes.
Figure 6. Antimicrobial activity results.
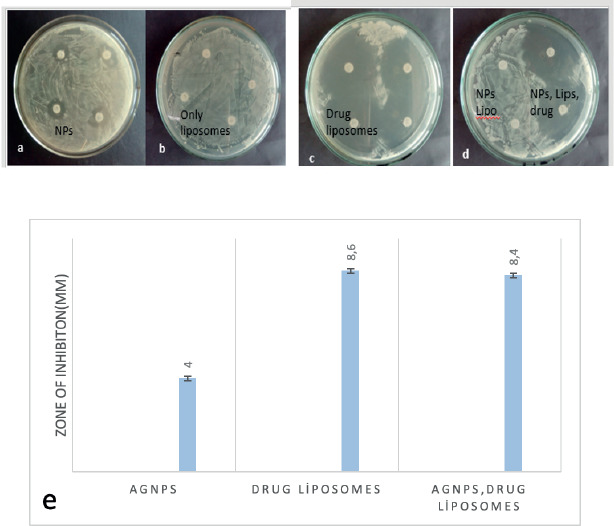
A report similar to the current study showed that the addition of metal NPs reduces the size of liposomes, therefore reducing the drug encapsulation efficiency and drug release effect (Torres et al., 2012). Similar observations were demonstrated by (Schumacher & Margalit, 1997), who performed antibacterial assay involving the samples of control liposomes, ampicillin encapsulated liposomes as well as free drug solutions by using paper disks against the test bacterium, Micrococcus luteus where liposomal formulations had lower MIC as compared to free drug. Drulis-Kawa et al., (2006) observed in vitro antibacterial efficacy of meropenem and gentamicin encapsulated liposomes against Gram negative and Gram positive strains of bacteria and the strategy was found effective. However, in contrast to the current study, the incorporation of silver nanoparticles in nano liposomes was also studied by Husseiny and co-workers and observed increased antibacterial activity against Escherichia coli and Staphylococcus aureus (Husseiny et al., 2015). In one of the study, the presence of silver nanoparticles stabilized the nanoliposomes by narrowing their size and reducing the size of the zone and similar were the findings of the current study. However, the incorporation of silver nanoparticles did not alter the zeta potential values of liposomes.
Conclusions
Conclusively, in the present study, silver nanoparticles were synthesized from Aspergillus niger BTCB01. In order to enhance the efficiency of the drug delivery system, AgNPs were added to drug liposomes. But the addition of AgNPs altered the stability of liposomes and encapsulation efficiency of the drug, which overall affected the antimicrobial activity. The system can be made efficient by optimizing different parameters, and it can be further studied in vivo to obtain the efficiency of the drug delivery system in animal models.