Introduction
Ferritin is often increased in acute inflammatory conditions. It is also known to act as an acute-phase reactant and contribute to immune dysregulation in severe cases of COVID-19. This suggests that ferritin may not only indicate the presence of inflammation but also actively participate in the cytokine storm observed in severe forms of COVID-19 (Kappert et al., 2020). The interaction between ferritin and cytokines may involve intricate feedback mechanisms in regulating pro-inflammatory and anti-inflammatory mediators. Cytokines can stimulate ferritin expression and vice versa; ferritin can also induce the expression of both pro- and anti-inflammatory cytokines (Ruddell et al., 2009).
Highly elevated levels of ferritin are a hallmark of hyperserotonemia syndromes, which include several severe medical conditions, such as macrophage activation syndrome, catastrophic antiphospholipid syndrome, adult-onset Still’s disease, and septic shock (Colafrancesco et al., 2020). Generally, high ferritin levels in hospitalised patients are associated with poor prognosis (Shakaroun et al., 2023). In addition, studies have linked elevated levels of ferritin and other pro-inflammatory markers in COVID-19 with negative outcomes (Shakaroun et al., 2023). Consequently, researchers are investigating various anti-inflammatory biological agents to control the strong immune response in COVID-19 patients. However, whether ferritin can be used as a reliable predictor of outcomes in COVID-19 patients is yet to be determined.
A study conducted on a sample of 20 COVID-19 patients revealed interesting findings regarding serum ferritin levels in individuals with varying degrees of COVID-19 severity (Cheng et al., 2020). The results of this study indicated that patients with severe and very severe COVID-19 exhibited higher levels of serum ferritin than those with mild or moderate disease. Specifically, the group of patients with very severe COVID-19 had significantly higher serum ferritin levels than those with severe COVID-19, with a median of 1006.16 ng/mL versus 291.13 ng/mL, respectively (Vargas-Vargas & Cortés-Rojo 2020). These findings suggest that serum ferritin levels could help identify COVID-19 patients with severe disease and monitoring disease progression. Additionally, elevated levels of ferritin in COVID-19 patients could indicate the presence of a severe secondary bacterial infection and may indicate poor prognosis (Vargas-Vargas & Cortés-Rojo 2020).
The measures of ferritin levels in patients infected with COVID-19 may is crucial for disease prediction. Hyperferritinemia, often accompanied by a cytokine storm, is especially noticeable in acute COVID-19 patients with other inflammatory factors (Hadi et al., 2022). It is well established that the study of ferritin is a current issue across various fields. Among these, ferritin serves as a potential prognostic marker for individuals infected with COVID-19 (Para et al., 2022). Taking this into account, our goal was to study ferritin, D-dimer and CRP in patients with COVID-19.
Materials and methods
The study sample consisted of 318 patients with COVID-19. In addition to biological material, the patient’s anamnesis, discharge and death epicrisis were studied. As about the comorbidities, from patients: Arterial hypertension: women: 40%; men: 57%; insulin-dependent diabetes: 33% women; men: 60%; heart disease: women: 28%, men: 79%The gold standard polymerase chain reaction (PCR) method was used to detect COVID-19 infection. It is performed in single, high-tech laboratories. Nasopharyngeal swabs were used as the test material.
Blood serum was used as the test material for ferritin determination. (Analyser name: BECKMAN COULTER. Reference value: 10-150; M.20.9-350.0 ng/ml; Number of patients: 318; Blood plasma was used as the test material for D-dimer determination. Blood plasma was used as the test material. The turbidometer method was used to determine the amount of D-dimer (Name of the analyser: Finecare Wondfo Fluorescent Immunochromatographic Analysis System. Reference value: 0-500; Number of patients: -310); C-reactive protein was evaluated using immunoturbidimetry methods on a modern automated analyser, the Cobas c311 from Roche. The number of patients: 318.
Statistical analysis was performed using GraphPad Prism 10. version program. The following tests were used: 1. Column descriptive statistics, t-tests; P < 0.05 was considered statistically significant.
Results and discussion
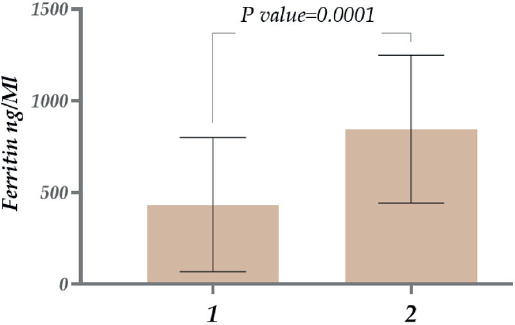
As mentioned above, elevated ferritin levels detected in COVID-19 patients correlated with the disease’s severity. Thus, ferritin is essential for assessing the disease’s ongoing course and the correct treatment management. (Gómez-Pastora et al., 2020). Therefore, we studied the ferritin (FRT) level in patients infected with COVID-19. In the studied population, the levels were ~1.8 times higher among male patients than in female patients (Figure 1).
Figure 1. Ferritin level in patients with COVID-19 (1. Females; 2. Males).
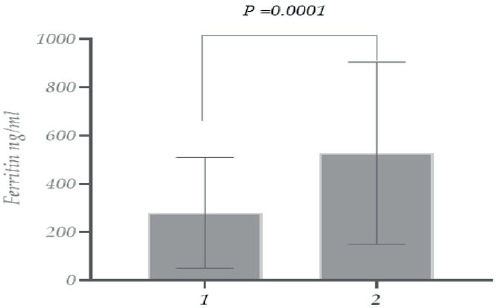
As for ferritin levels in the case of recovered and deceased female patients, the levels of ferritin were ~ 3 times higher in patients with lethal outcomes (Figure 2).
Figure 2. Study of ferritin count with COVID-19 (Female patients) (1. recovered; 2. deceased).
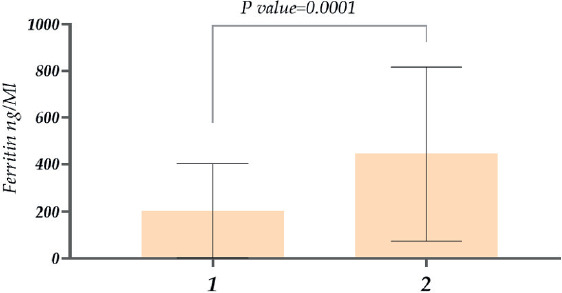
Regarding ferritin levels in men, the concentration was ~1.9 times higher in deceased male patients than in recovered patients (Figure 3). In addition to comparing genders, we also studied the ferritin level variations in different age groups. The research showed that in the case of 11-20-year-olds, ferritin was nearly ~1.3 times higher compared with younger patients between 1-10 years old. Similarly, in 21-30-year-old patients, ferritin levels were approximately 5.5 times higher compared with 11-20-year-olds. Considering patients aged 31-40 years, ferritin levels were ~1.3 times higher than in patients aged 21-30 years. A relatively low ferritin level was observed in 41-50-year-old patients, then there was an increase again in patients aged 51-60 years old. As for 71-80 year old patients, it was ~1.7 times higher than in 61-70 year old patients. And finally, the highest level of ferritin was detected in the case of 81-90-year-old patients. It is also noteworthy that a statistically significant difference was revealed between the 11-20 and 21-30 age groups when other age groups (P=0.0001)
Figure 3. Study of ferritin levels in patients with COVID-19 (male patients) (1. recovered; 2. deceased).
The study of ferritin among recovered patients (both female and male patients) showed that, in the case of surviving patients, the level of ferritin was consistent across all age groups (51-60 years: 310.5, 200; 61-70 years: 312.8, 207; 71-80 years: 312.8, 191.4; 81-90 years: 294, 217.2) (Figure 5A). In contrast, for deceased patients, the level of ferritin increased by 2.3, 2.46, 2.6, and 2.4 times compared to the corresponding age groups of surviving patients (Figure 5A and B). Notably, the ferritin level was highest in patients aged 71-80 years (Figure 11B). The inflammation during COVID-19 may represent part of the spectrum of hyperinflammatory syndromes. Furthermore, it is plausible that a common underlying pathogenic background could be successfully used to develop therapeutics targeting inflammatory mediators. (Colafrancesco et al., 2020). Infected patients may benefit if ferritin plays a pathogenic role as a mediator of COVID-19, thereby reducing ferritin and cytokine levels in SARS-CoV-2. Thus, ferritin monitoring during hospitalisation helps to identify severe patients/the possibility of controlling disease progression/ possibly predicting clinical deterioration (Gómez-Pastora et al. 2020).
Figure 4. Ferritin level in patients with COVID-19 (different age groups).
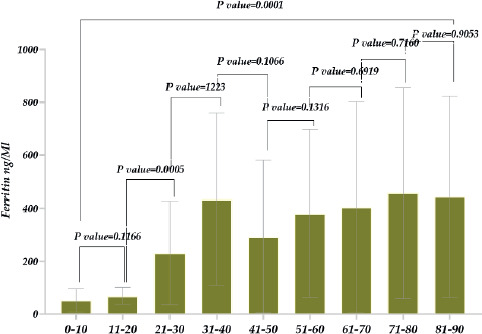
Figure 5. Ferritin level in patients with COVID-19 (different age groups) A. Recovered patients b. Deceased patients.
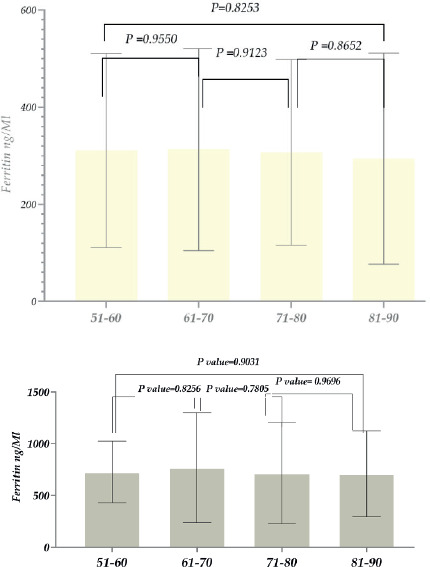
Figure 6. A D-dimer (DD) level in patients with COVID-19. A (1. females; 2. males); B, D-dimer (DD) levels in patients with COVID-19 -Males (1. recovered; 2. deceased); C, D-dimer (DD) levels in patients with COVID-19- recovered (1. females; 2. males); D, D-dimer (DD) levels in patients with COVID-19- deceased (1. females; 2. males).
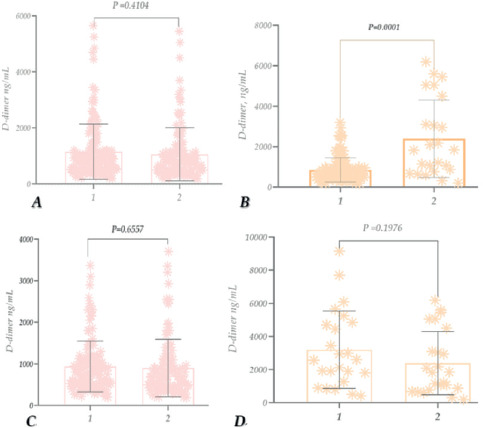
Figure 7. D-dimer (DD) levels in patients with COVID-19 recovered (A); D-dimer (DD) levels in patients with COVID-19 – deceased (B).
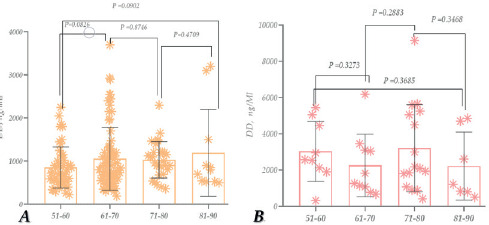
Figure 8. CRP study in patients with COVID-19 (1. females, 2. males) (A); CRP study in patients with COVID-19 – Females (1. recovered 2. deceased) (B); CRP study in patients with COVID-19 – males (1. recovered 2. deceased) (C); CRP study in dead patients with COVID-19 (1. females, 2. males) (D); CRP study in recovered patients with COVID-19 (1. females, 2. males) (E).
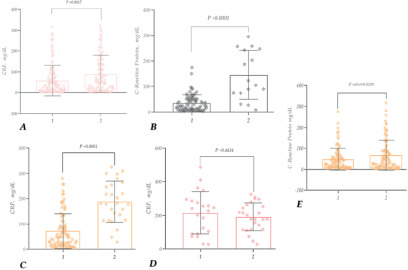
Figure 9. CRP study in patients with COVID-19 -recovered (females, males) (A); CRP study in patients with COVID-19 -deceased (females, males) (B).
Considering the above, we also studied the D-dimer level in the case of patients suffering from COVID-19, along with ferritin. As the study results showed, the D-dimer level compared to its reference level was ~2 times higher within COVID-19 patients. It should also be noted that the levels were similar among both female and male patients (Figure 6A).
As for the D-dimer levels in recovered and deceased female patients, the D-dimer level was high in both cases, ~1.8 and 4.6 times higher than the reference norm. As for the difference between recovered and deceased patients, the level in deceased patients increased ~4.1 times (compared to recovered patients) (Figure 6 B). In the case of male patients, the D-dimer levels were also high, ~1.7 times and 4.7 times higher than the reference level. The level was ~2.8 times higher in deceased patients than in recovered patients (Figure 6C). The level of D dimer in recovered patients (Female and male patients) was almost the same, and it increased ~1.8 times compared to the reference norm (Figure 6D.); in deceased patients (female and male patients) its level increased by ~6.4 and ~4.7 times compared to normal (Figure 6D).
The study of D-dimer at different ages showed that its level was high (compared to the reference level) in all age groups: 51-60 years (~1.7), 61-70 years (~2.11), 71-80 years (~2.064), and 81-90 years (~2.37). However, the rate was highest in the 81-90 years age group (Figure 7A). For deceased patients, the level was exceptionally high compared to recovered patients in all age groups (3039 ng/ mL, 2256 ng/mL, 3209 ng/mL, and 2220 ng/mL). However, it was particularly high in 71-80-year-old patients. Those 61-60 years old are noteworthy (Figure 7 B.).
According to the study, the CRP level increased by approximately ~9.6-Times and 14 times compared to the reference norm. It is also noteworthy that the level was higher in male patients (Figure 8A). The CRP level, compared to its reference level, increased ~5.8-times for recovered patients and around ~24.4 times for deceased patients (female patients). Additionally, it increased by about 4.1 times in the case of deceased patients compared to healthy patients (Figure 8B).
In the case of male patients, the CRP level in recovered patients was ~11.6-times higher, and ~31.3 times higher in deceased patients, compared to the reference norm. Regarding the difference between recovered and deceased patients, the CRP level increased by ~2.6-times (Figure 8C). When analysing the CRP levels of deceased patients, their rate had increased compared to the reference, especially in female patients (Figure 8D).
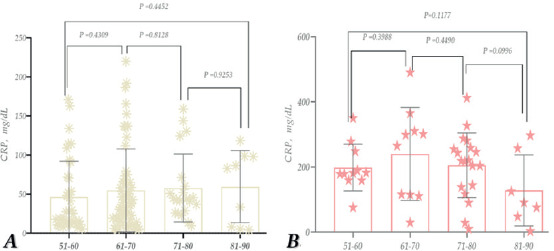
No statistically significant difference was observed in comparing the CRP indicator between age groups in recovered patients (Figure 9A); however, it was detected in deceased patients (Figure 9B). In patients who died at the ages of 81-90, 71-80, 61-70, and 51-60, the CRP levels were ~4.3, 4.4, 3.5, and 2.1 times higher, respectively, compared to those who had recovered (compared to the corresponding age groups).
Thus, according to the study, the CRP level is elevated in patients infected with COVID-19, especially in the case of deceased female patients. When comparing age groups, the significant representation of individuals aged 61-70 is noteworthy. In patients, this age group was also notably high compared to all other age groups and recovered patients of their corresponding age (Figure 9).
The study supports the idea that high ferritin levels indicate a poor prognosis for COVID-19 patients. Zhou et al. (2020a) conducted a retrospective, multicentre cohort study to examine the clinical course of COVID-19 patients, including laboratory biomarkers. The study included a total of 191 patients, of whom 137 were discharged (survivor group) and 54 died (deceased group) while in the hospital. The findings revealed that serum ferritin levels were significantly higher in non-survivors compared to survivors at the time of admission (median: 1435.3 vs. 503.2 µg/L, p<0.0001). Furthermore, the median serum ferritin levels exceeded the upper limit of detection after day 16 of hospitalisation in these patients, indicating a continuous increase in ferritin levels. The results suggest that serum ferritin is a valuable biomarker for predicting clinical outcomes in patients with COVID-19. The elevated levels of ferritin observed in non-survivors may indicate that these patients are more susceptible to developing an inflammatory storm linked to severe illness and mortality in COVID-19. The study provides important insights into the role of ferritin in COVID-19 pathogenesis and emphasises the significance of monitoring this biomarker in patients with COVID-19 (Zhou et al., 2020a; Carubbi et al., 2021). Chen et al. (2020) conducted a retrospective study on 99 COVID-19-infected patients, out of which 63 showed significantly elevated serum ferritin levels beyond the normal range during the infection (Chen et al., 2020). Additionally, autopsies of 12 patients who died from SARS-CoV-2 infection also exhibited elevated ferritin levels (Fox et al., 2020). Several studies have investigated the relationship between ferritin levels and comorbidities in patients with COVID-19. One such study aimed to investigate the impact of diabetes mellitus as a risk factor contributing to the poorer prognosis of COVID-19. The study collected and analysed data from 174 consecutive patients with confirmed COVID-19. The patients were divided into diabetic and non-diabetic cases based on their medical history, with 37 diabetic cases and 137 non-diabetic cases. To ensure that no biases are present in the study due to other medical factors, patients with pre-existing conditions apart from diabetes were not considered to be a part of this research. The assessment revealed that cases had increased levels of ferritin compared with controls (764.8 vs. 128.9 µg/L), respectively (p < 0.01). The investigation found similar disparities in ESR levels, D-dimer, CRP, and IL6. While patients with other pre-existing conditions also had elevated serum ferritin levels, these increases were not as significant. Furthermore, it concluded that activation of the monocyte-macrophage system, contributing to an inflammatory storm, can be demonstrated through elevated ferritin levels. These results suggest that cases were more prone to hyperinflammatory response, potentially leading to rapid deterioration and a poor outcome for COVID-19-infected patients (Guo et al., 2020). Moreover, there is an association between ferritin levels and severe, life-threatening COVID-19 cases. Liu et al. (2020) analysed peripheral blood samples from patients with advanced COVID-19 and compared them with non-advanced COVID-19 controls. An advanced level of infection was indicated by elevated ferritin levels, demonstrating a close association (Liu et al., 2020). Moreover, laboratory findings showed an immune system over-activation with elevated inflammatory biomolecules, including ferritin (Mehta et al., 2020). Although most studies found a strong association, some studies contradict significant findings on ferritin levels and COVID-19 severity. Feld et al. reported that levels of ferritin obtained throughout the disease or at specific durations were not a strong predictor of evaluated outcomes, including all-cause mortality, new mechanical ventilation, and the need for kidney transplantation. Based on this study’s data, it can be concluded that while higher ferritin levels are linked with deaths throughout the course of an disease, they cannot be used as a reliable predictor for several significant outcomes, such as death. (Feld et al., 2020). Since the outbreak of COVID-19, D-dimer has become a potential biomarker for prognosis and treatment management in clinical settings. Elevated D-dimer levels and normal fibrinogen levels are a hallmark of coagulopathy in COVID-19. This condition is associated with high inflammation during COVID-19 illness, which can lead to mortality and morbidity due to thrombotic complications.
In severe or critical COVID-19 patients, D-dimer levels are significantly higher than in mild/moderate patients. This suggests a markedly high inflammation and consumptive coagulation state (Liu et al., 2020). D-dimer levels can help predict the risk of complications in COVID-19 patients, such as venous thromboembolism (VTE). By measuring D-dimer levels at admission, healthcare professionals can identify patients at high risk for developing VTE and strategically manage them to prevent further complications (Nauka et al., 2021). Several studies have found that high levels of D-dimer at admission are associated with poor outcomes and in-hospital mortality in COVID-19 patients (Poudel et al., 2021).
Tang et al. conducted a retrospective analysis and reported that deceased COVID-19 patients had higher levels of D-dimer, fibrinogen degradation products (FDP), and prothrombin time (PT) at the time of admission than those who survived the disease. D-dimer levels can serve as an essential biomarker for predicting the risk of complications and mortality in COVID-19 patients. By monitoring and managing D-dimer levels, healthcare professionals can improve patient outcomes and prevent further complications. In non-survivors, a marked increase in D dimer levels (> 15.0 μg/mL) and decreasing levels of antithrombin III are associated with progressive consumptive coagulopathy and critical disease (Tang et al., 2020). Additionally, high D dimer levels (>1 μg/mL) have been linked to a significant increase in mortality (18 times higher) compared to those with lower levels among COVID-19 patients (Zhou et al., 2020b). Moreover, patients with high D dimer levels (>3 μg/mL) are unresponsive to prophylactic treatment for VTE (Maatman et al., 2020). Patients with COVID-19 pneumonia are at high risk of developing acute pulmonary embolism (ARE) if they have elevated levels of D-dimer, which is a serious condition associated with mortality (Santos-Poleo et al., 2020). Although the pathophysiology behind the development of ARE in COVID-19 patients is still being studied, all patients with elevated D-dimer levels should undergo Computed Tomography Pulmonary Angiography (CTPA) to rule out this clinical suspicion and initiate early treatment. A study by Sakka et al. recommended using D-dimer levels to sort COVID-19 patients at the time of admission to efficiently manage clinically severe patients (Sakka et al., 2020). Monitoring its levels can facilitate timely treatment initiation for high-risk patients. Additionally, D-dimer testing is a cost-effective laboratory procedure. Initially known to cause only respiratory distress and illness, it has now been ascertained that the virus can affect any organ and lead to organ dysfunction. There have been several studies to find the most suitable biomarkers reflecting the disease severity and predicting the prognosis. As the infection is a hyperinflammatory state, several markers that reflect the inflammatory state in the body have risen in these patients. A cytokine storm is the appropriate word for the surplus increase in the markers, leading to complications in multiple organs (Ceci et al., 2022). Although, why some patients fall critically ill while others don’t remain a topic of great interest and an unsolved puzzle. But, out of many markers, three important ones that might predict the severity of the disease are: CRP, Ferritin, and D-Dimer. These markers can increase in the blood within 6-10 hours of any tissue damage (Black et al., 2004). There is mounting evidence that these can be used as diagnostic and prognostic markers; hence, hospitals have also used these in their practice accordingly when measured at the right time. These markers can help in proper resource allocation and management if critical patients, especially in the context of respiratory support (Wang et al., 2020; Gao et al., 2020; Linarez Ochoa et al., 2022).
Acute phase proteins or reactants (APRs) are a class of proteins that increase or decrease during the process of inflammation. Physiologically, the level of positive APRs is higher than the negative APRs. Positive APRs include Albumin, Transferrin, etc., and negative APRs include CRP, Fibrinogen, Ferritin, Procalcitonin, etc. During inflammation, where normal physiology is disrupted, negative ARPs go up and hence can be used as a marker for an inflammatory state. CRP is an acute-phase reactant produced by the liver that can get elevated in multiple inflammatory, autoimmune, and cardiovascular conditions (Sproston and Ashworth, 2018). D-dimer is a fibrin degradation product that signifies breakdown of clots in the body. Studies have reported that COVID-19 infection can induce formation of microthrombi in different vessels of the body, especially that of the lungs and the brain (Luo et al., 2020). 3.75–68.0% of the COVID-19 patients have been found to have raised D-dimer levels (Wu et al., 2020). Ferritin is an iron storage molecule generally, but like CRP also has a role on an acute phase reactant. Haematological changes occurring in COVID-19 were demonstrated. As per the meta-analysis performed by Huang et al (2020) elevated serum CRP was associated with an increased composite poor outcome [RR 1.84 (1.45, 2.33), p < 0.001; I2: 96%, p < 0.001] that included severe COVID-19 [RR 1.41 (1.14, 1.74), p = 0.002; I2: 93%, p < 0.001], need for ICU care [RR 1.96 (1.40, 2.74), p < 0.001], but not mortality [RR 2.95 (0.90, 9.68), p = 0.07; I2: 99%, p < 0.001] (Huang et al., 2020). Elevated D-dimer reflected increased mortality amongst those patients. In 10 studies, it was found that hyperferritinemia was seen in fatal cases rather than just severe cases (Huang et al., 2020). It is also important to denote that besides ferritin, D -dimer and CRP, there are some other potential biomarkers and potential targets for the treatment of Sars-CoV-2 that may be also applied and used to predict severity of SARS-CoV-2 infection, especially the ones associated with ferritin, D-dimer, CRP, and ferritin, and inflammatory mediators. For example, some miRNAs that were initially described in various tumor models, such as miR-19a-3p/19b-3p, and miR-92a-3p or miR-16-5p (Feng et al., 2014; Ghafouri-Fard et al., 2022) are described with a good potential to be used as biomarkers for early stage of SARS-CoV-2 infections, as well as reviewed and listed by Ergun et al. (2023) such as miR-146a/155/18a-5p/133/21-5p also linked with the disease severity (Ergun et al., 2023). For example, miR-92a-3p was especially found to be decreased in critically ill patients (De Gonzalo-Calvo et al., 2021). The same authors, observed association between miRNAs miR-92a-3p, miR-16-5p, miR-451a/486-5p/150-5p with ferritin and D-dimer, while CRP was found to be in a correlation with miR-27a-3p/27b-3p/451a/148a-3p/491-5p and miR-199-5p (De Gonzalo-Calvo et al., 2021) indicating that miRNA up or downregulation and targeting may be one of the mechanisms for additional treatment of SARS-CoV-2. Another study regarding liver function of COVID-19-infected patients revealed that the high creatinine levels are associated with the severity of COVID-19 disease (Murvanidze et al., 2023).
Thus, the study of ferritin in patients suffering from COVID-19 (using the population of Adjara as an example) revealed a significantly higher ferritin level than the reference level in the diseased population. A relatively high level of ferritin was detected in the samples of men compared to women. Regarding the level of ferritin in the deceased, a ~3 times and a ~1.9 times higher level was detected in female patients compared to those who recovered, and in men, respectively. As for the highest level of ferritin in the case of dead patients, the latter was observed in patients aged 81-90 years. An increased level of D-dimer compared to the reference level was detected; Its level is especially elevated in men; And as for its amount in deceased patients, it has increased ~4.1 times; D-dimer level is significantly increased at the age of 71-80 years; The level of CRP is ~5.8 times higher compared to the reference level; Moreover, CRP level increased ~24.4 times in case of dead women; In particular, according to the comparing age groups, a high level of CRP was observed in 61-70 years’ patients. Future treatment modalities should not only rely on symptomatic therapy but also gene therapy, specifically modulating molecules such as miRNAs.
Conclusions
Our study revealed that the diseased population exhibited significantly higher ferritin levels. The ferritin levels in patients with lethal outcomes were considerably higher than those in patients who successfully recovered. Thus, the findings reveal a noteworthy elevation in D-dimer levels, particularly in men and deceased patients, with a notable elevation in the 71-80 age group. Additionally, CRP levels were markedly higher, especially among deceased women and individuals aged 61-70.