Introduction
Tissue engineering is based on three key factors: cells, growth factors, and tissue scaffolds, and it is a discipline aimed at functionally restoring damaged tissues. Tissue scaffolds are regenerative structures that support tissue repair and are significant as they are used in the healing of damaged tissues and organs. They support cells when seeded in vitro and promote matrix formation, laying the foundation for tissue transplantation (Howard et al., 2008).
The materials used in the production of tissue scaffolds play a crucial role in promoting cell proliferation and differentiation. An ideal tissue scaffold should possess good biocompatibility, appropriate pore size and porosity, and excellent mechanical strength, while also exhibiting characteristics suitable for the specific area of the body where it will be applied (Keçeciler et al., 2023). Tissue scaffolds fabricated via the electrospinning technique have garnered significant attention in tissue engineering due to their excellent biocompatibility, optimal biodegradability, non-toxic nature, micro-to nano-scale porosity, and their ability to achieve a high surface-to-volume ratio (Sadeghi et al., 2018; Cam et al., 2019). Tissue engineering has garnered significant attention in recent years (Aydogdu et al., 2019). Polymer/ceramic composite biomaterials and tissue scaffolds produced using the electrospinning technique exhibit enhanced mechanical strength and optimal cell adhesion. The superior performance of composite materials has led to a surge in research focusing on polymer and ceramic materials (Hoque et al., 2014).
Polymer materials provide a suitable framework for replicating the natural architecture of soft tissues. Integrating bioceramics into polymers enhances their potential for repair and regeneration strategies (Zou et al., 2018; Filippi et al., 2020; Jazayeri et al., 2020). PVA, a synthetic polymer, is both biocompatible and biodegradable; however, its limited cell recognition sites reduce its bioactivity. In contrast, GEL offers numerous integrin binding sites, facilitating cell adhesion and differentiation (Heydary et al., 2015; Perez-Puyana et al., 2018). Bioceramics are widely favored for incorporation into tissue scaffolds due to their ability to support cell growth and proliferation, exhibit antimicrobial properties, contribute to the repair of bone and dental tissues, and serve as effective materials for soft tissue healing applications (Xie et al., 2019; Zhang et al., 2021). Chemical precipitation, the most commonly used technique for bioceramic synthesis, enables the production of bioceramics with high efficiency and low cost in the absence of organic solvents. Moreover, the only by-product of the reaction is water, which does not contain any other elements, making this method quite suitable for biomedical applications ( Mazumder et al., 2019; Santhosh et al., 2013; Gunduz et al., 2013; Şahin et al., 2018).
In the present study, it is aimed to produce a composite tissue scaffold that mimics the extracellular matrix (ECM). For this scaffold, which was fabricated using both ECM composite components and nanoscale production, the following steps were carried out: synthesizing bioceramics from sea snail shells via chemical precipitation, incorporating the synthesized bioceramics into a PVA/gelatin (GEL) solution, and producing tissue scaffolds with suitable cell adhesion and biocompatibility via electrospinning technique. Additionally, the structural, morphological, mechanical, and thermal characterizations of the synthesized bioceramics and the fabricated tissue scaffolds were performed to evaluate their properties for tissue engineering applications.
Materials and methods
Materials
For the synthesis of bioceramics, marine snail shells were obtained from a seafood company in Eminönü, Istanbul. For tissue scaffold production, a polyvinyl alcohol (PVA) polymer with a molecular weight of 85.000-124.000 g/mol was purchased from Sigma-Aldrich (Turkey). Food-grade gelatin(GEL) was acquired from a local herbalist in Eminönü, Istanbul. Ultrapure water with a resistance of 18.3 MΩ was used as the solvent for preparing the polymer solution.
Bioceramics synthesis
Marine snail shells were selected as the source for bioceramic synthesis. Initially, the shells were sterilized in an ultrasonic bath at 90°C for 30 minutes, followed by drying in an oven at 100°C for 3 hours to ensure purification. The dried shells were then ground into powder using a ceramic mortar and further sieved through a stainless steel mesh with a size of 100 µm to achieve a uniform particle size.
Thermal analysis was conducted to quantify the calcium carbonate (CaCO3) content in the powders. Using this data, the required amount of phosphoric acid (H3PO4) was calculated based on the stoichiometric (molar) calcium-to-phosphorus (Ca/P) ratios: 1.67 for hydroxyapatite and 1.50 for tricalcium phosphate. For each batch, 2 g of powdered shell was measured and transferred into a beaker containing 50 ml of pure water. The mixture was heated to 90°C on a magnetic stirrer, and the calculated H3PO4 solution was added dropwise. Once bubbling ceased, the mixture was continuously stirred at 90°C for 8 hours and then allowed to cool to room temperature to complete the reaction. The resulting precipitate was separated by centrifugation, and the wet powder was dried in an oven at 100°C for 24 hours. The dried precipitate was sintered at 850°C for 4 hours to obtain the final bioceramic product (Sahin et al., 2018; Yelten and Yılmaz, 2010).
Preparation of solutions
To produce composite tissue scaffolds, PVA, GEL, and bioceramic powders were dissolved in distilled water using a magnetic stirrer. A pure 10:0 (PVA: GEL) solution was prepared by dissolving 10 g of PVA in 100 ml w/v of distilled water and stirring at 80°C for 150 minutes. Subsequently, GEL was added to the 10:0 (PVA: GEL) solution in weight ratios of 1% and 3% w/v. These mixtures were stirred at 40°C for 150 and 180 minutes, respectively, to obtain 10:1 (PVA: GEL) and 10:3 (PVA: GEL) solutions (Linh et al., 2010). Finally, 1% bioceramic powder was added to the 10:1 (PVA: GEL) solution and stirred at 37°C for 210 minutes, resulting in the 10:1:1 (PVA:GEL:Bioceramics) solution (Song et al., 2010; Ba Linh et al., 2013). The preparation parameters for the solutions required for composite tissue scaffold fabrication are shown in Table 1.
Table 1. Preparation parameters of solutions used for the fabrication of composite tissue scaffolds.
| Polymer weight ratio | Temperature (°C) | Time (min) |
|---|---|---|
| 10:0 (PVA: GEL) | 80 | 150 |
| 10:1 (PVA: GEL) | 40 | 150 |
| 10:3 (PVA: GEL) | 40 | 180 |
| 10:1:1(PVA:GEL:Bioceramics) | 37 | 210 |
1.1. Production of composite tissue scaffolds
Composite tissue scaffolds were fabricated using the electrospinning technique by applying the working parameters listed in Table 2 (Sengor et al., 2018). To remove any residual solvents, the produced tissue scaffolds were dried in an oven at 45°C for 24 hours.
Table 2. Electrospinning production parameters for composite tissue scaffolds.
| Polymer Content | Flow Rate (ml/hr) | Distance (cm) | Voltage (kV) |
|---|---|---|---|
| 10:0 (PVA: GEL) | 5.0 | 15 | 30 |
| 10:1 (PVA: GEL) | 5.0 | 15 | 30 |
| 10:3 (PVA: GEL) | 5.0 | 10 | 25 |
| 10:1:1(PVA: GEL:Bioceramics) | 3.0 | 5 | 25 |
1.2. Characterization studies
Fourier transform infrared (FTIR) analysis: The presence of functional groups in the bioceramics and composite tissue scaffolds was determined by FTIR spectroscopy (FT/IR-6600, JASCO, Tokyo, Japan) in the wavelength range of 400-4400 cm-1. These analyzes enabled the structural characterization of the bioceramics and composites, confirming their composition and the successful incorporation of bioceramics into the composite tissue scaffolds.
Field emission gun scanning electron microscopy (FEGSEM) analysis: Bioceramics and composite tissue scaffolds were coated with gold-palladium under Argon (Ar) gas and analyzed using a field emission gun scanning electron microscope (FEGSEM 450, FEI Quanta, Oregon, USA). Images were captured under high vacuum at a range of 12.00-15.00 kV potential, with scales from 100-500 nm to 500 nm-2 µm. The diameters of 40 nanofibers were measured from the images, their arithmetic mean was calculated, and the nanofiber diameter distributions were determined. For all the samples, morphological analysis was conducted at various magnifications.
Tensile test: The tensile test for composite tissue scaffolds was performed according to ASTM D882-10 standards using a tensile testing machine (DVT UZM K3, DVT DEVOTRANS, Istanbul, Türkiye). The test was conducted under a 500 N load, with a tensile speed of 5 mm/min.
Thermogravimetric analysis (TGA): The CaCO3 content of marine snail shells was determined using a TGA device (STA7200, Hitachi, Tokyo, Japan). Thermal characterization of the samples was conducted under Nitrogen (N2) atmosphere at a heating rate of 15°C /minute, within the temperature range of 30-900°C.
Results and discussion
Thermal characterization of bioceramics with TGA
Upon analysis of the TGA thermogram of sea snail shells (Figure 1), it was observed that 45.0% of the material was lost during heating, attributed to the decomposition of organic matter and impurities, while the remaining 55.0% was identified as inorganic CaO.
Figure 1. TGA thermogram of sea snail shells.
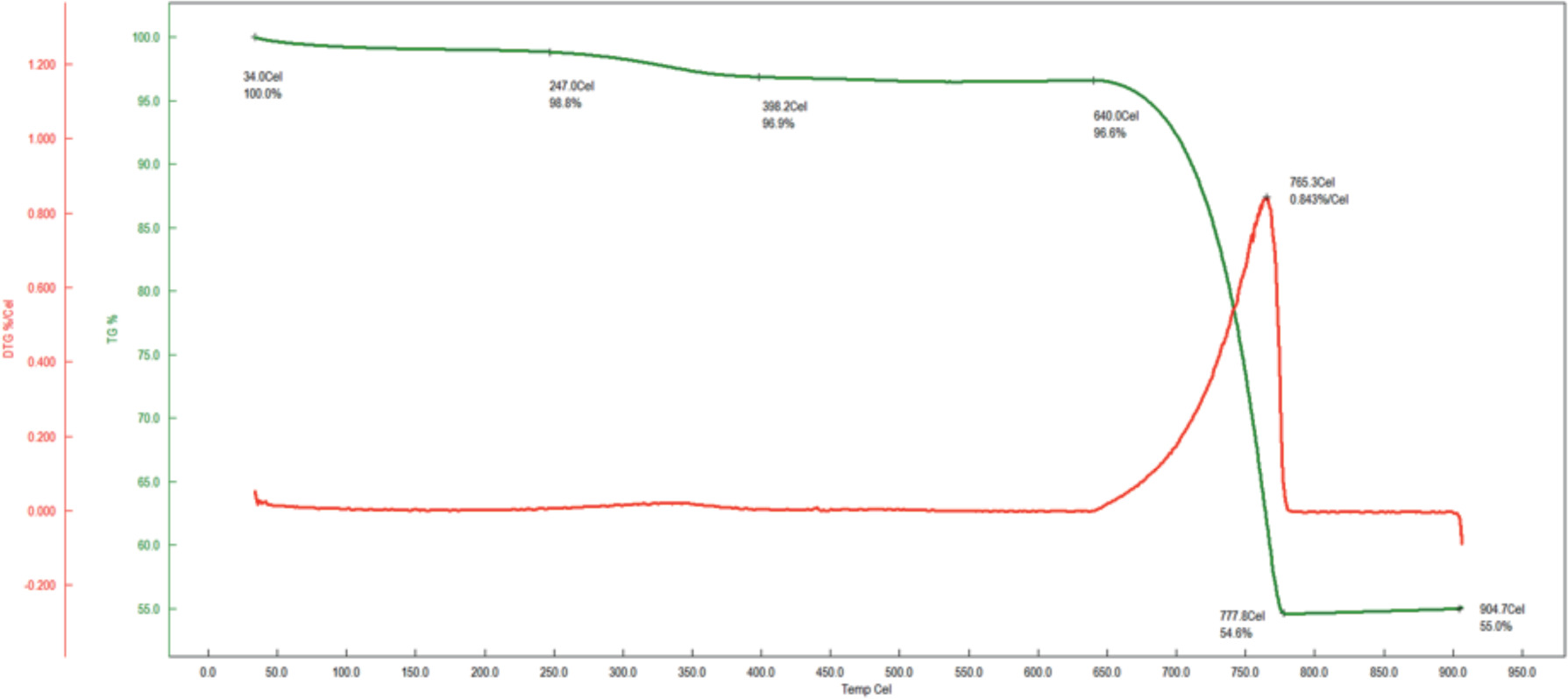
Using the 55.0% CaO content, the stoichiometric molar ratio corresponding to the mineral composition of bone in the human body was calculated to determine the required amount of H3PO4. This calculated H3PO4 quantity was subsequently incorporated3 into the CaO structure during the chemical precipitation process, enabling the successful synthesis of bioceramics.
Structural characterization (FTIR analysis)
The FTIR spectra of the bioceramics and tissue scaffolds are presented in Figure 2. In the analysis of the spectra, the functional groups of the bioceramic powders sintered at 850°C for 4 hours were confirmed.
Figure 2. FTIR spectra of bioceramics and scaffold composites.
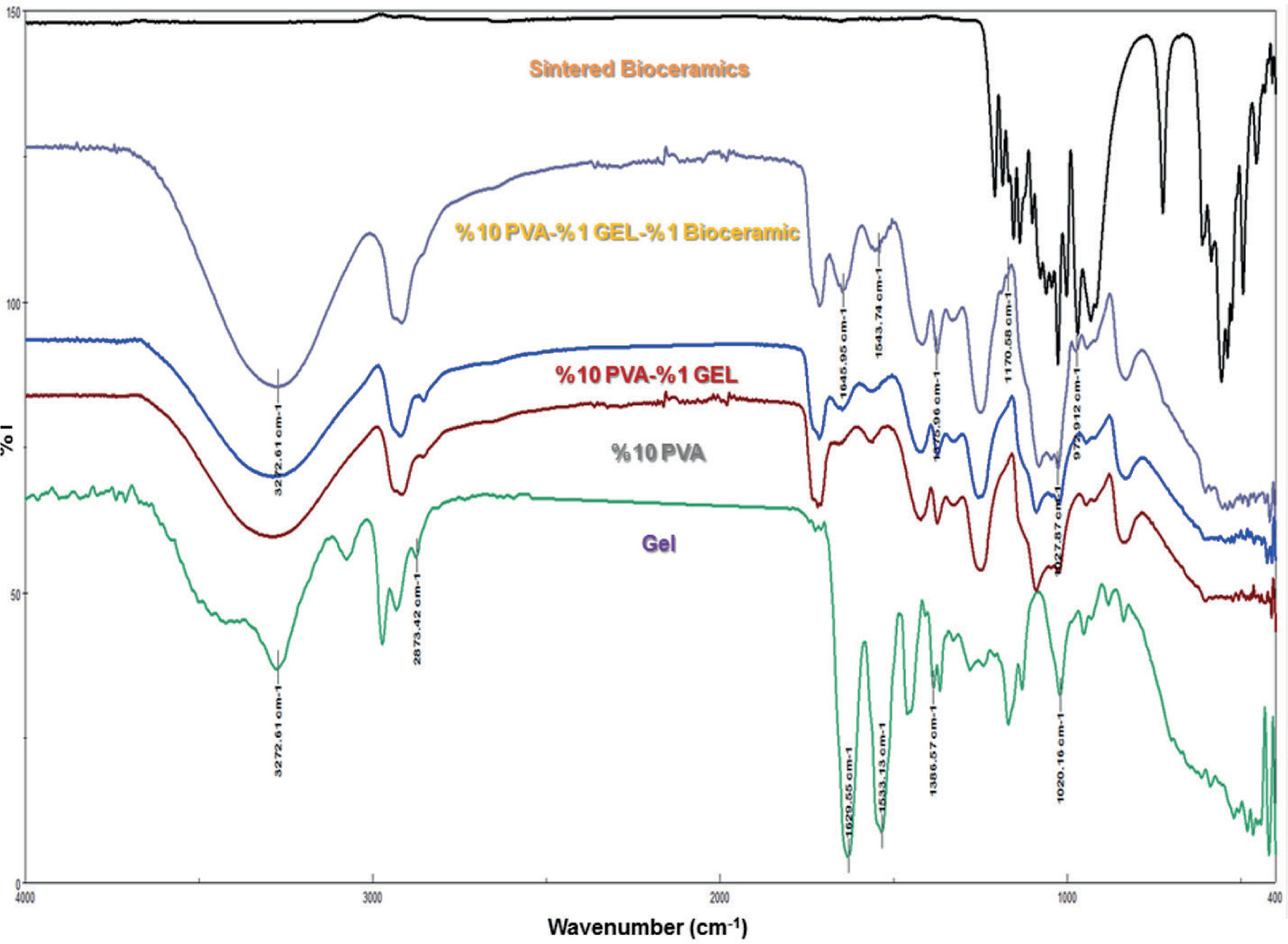
A phosphate group O–P–O bending mode was observed at 494, 528, 560, 609, 667 cm-1 and another bending band is detected at 471 cm-1 P–O asymmetric stretching mode bands of the phosphate group were at 973, 1031, 1066, 1101, 1139, 1187, 1211 cm-1. P–O–P symmetric stretching bands were obtained as medium intensity peaks at 726, 751 cm-1. Similarly, the phosphate group P–O symmetric stretching vibration band at 954 cm-1 and harmonic overtone were detected at 1156 cm-1. These findings can be attributed to the phospate group that consists of the hydroyapatite formulation of the synthesized bioceramic. On the other hand, the carbonate group function was also confirmed for the bioceramic with the following findings; a carbonate group stretching mode at 1415 cm-1 and a carbonate group bending mode at1456 cm-1. The characteristic peaks of PVA, the matrix of the composite, in the membrane were also observed. Hydroxyl (–OH) groups were indicated at 3272.61 cm-1, and the alkyl group bands were observed at 2854.13 cm-1 for (–CH3), 2916.81 cm-1 for (–CH2) functional groups. The peak at 835.02 cm-1 is due to the (-C-C-) vibrations. A weak peak at 1700-1750 cm-1, corresponding to the C=O (carbonyl group), can be due to residual acetic acid ester groups in PVA. The peak at 1718.26 cm-1 can be attributed to the hydrogen bonding between the carbonyl and hydroxyl groups in PVA chains, which occurs due to hydrophilic interactions (Hernández et al., 2024; Tüzün, 2023).
These bands can vary depending on the interactions of the composite parts. For example, the –OH vibration band of PVA was detected at 3272.61 cm-1. When 1% GEL was added to a 10% PVA solution, no specific GEL peak was observed; however, the position of the PVA peaks shifted, and their intensity increased. When examining the spectra of PVA and GEL, the –OH hydroxyl group at 3272.61 cm-1 was found in the same position for both polymers, which explains the superposition of the peaks. Nonetheless, the dominant effect of GEL on PVA caused the displacement of PVA peaks. This result confirms the successful formation of a composite material as explained in the similar studies in the literature (Bulus et al.,2020; Razzaq et al., 2021). The distinctive peaks of the functional groups of PVA, Gel and bioceramic materials are shown in Figure 2.
Morphological characterization by FEGSEM analysis
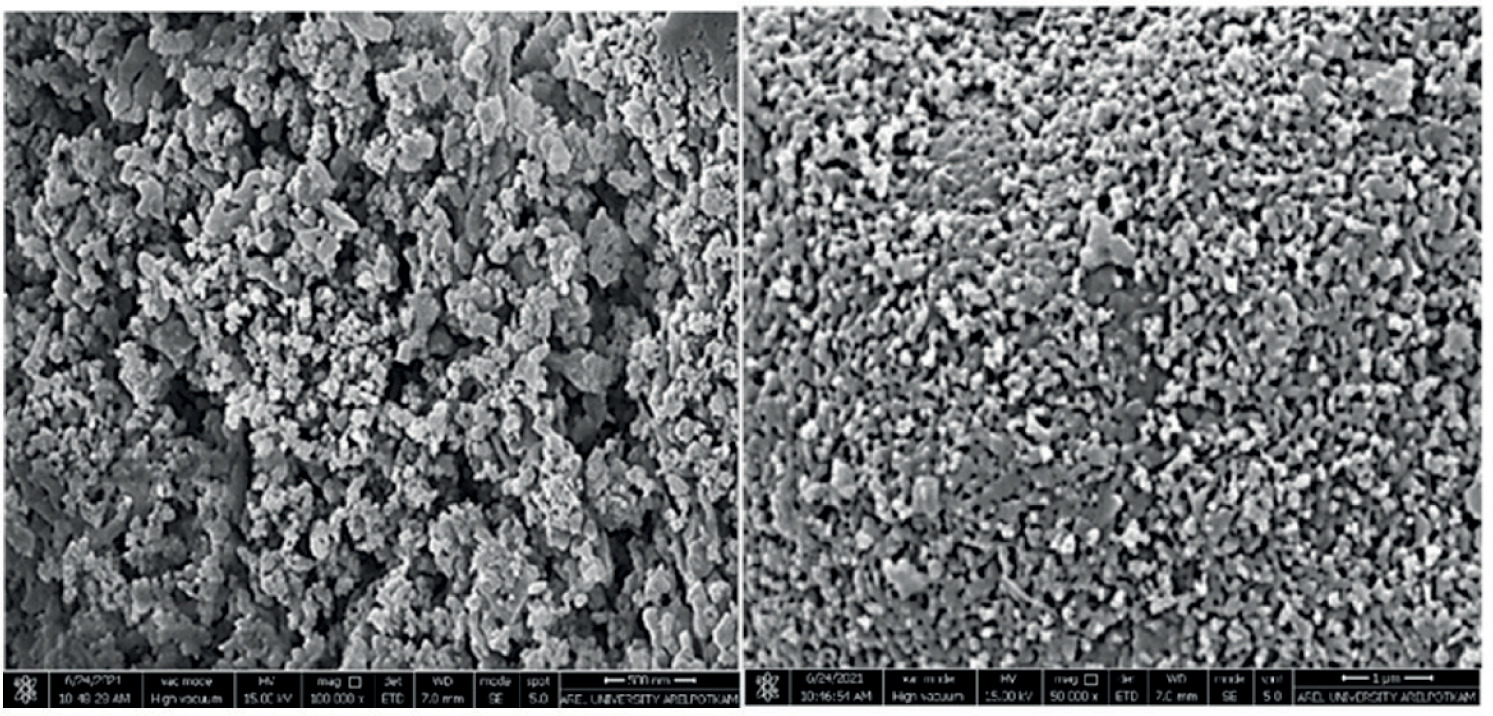
When examining the FEGSEM morphological images of bioceramic powders synthesized from sea snail shells and sintered at 850°C for 4 hours (Figure 3), particles with various allotropic structures, including spherical, cubic, and needle-like forms, were observed (Buluş, 2017). These diverse particles exhibit high bioresorbability and bioactivity for a bioceramic (Biesuz et al., 2021).
Figure 3. SEM Images of Bioceramic Powders Synthesized from Sea Snail Shells.
The FEGSEM morphological analysis of the scaffolds (Figure 4) revealed that the fiber diameters of the 10:0 (PVA: GEL) sample ranged between 92 and 205 nm. The incorporation of 1% GEL produced the thinnest fibers observed in the study, with diameters measured between 72 and 142 nm. While the addition of 1% GEL promoted significant fiber thinning. Increasing the GEL content to 3% caused a rise in solution viscosity, leading to thicker fibers with diameters ranging from 145 to 308 nm. Moreover, the incorporation of 1% bioceramic into the 10% PVA/1% GEL composite resulted in nanofiber scaffolds with fiber diameters in the range of 85 to 280 nm. Among all the samples, the 10:1 (PVA: GEL) composite exhibited the thinnest average fiber diameter (Gautam et al., 2021). The mean fiber diameters for each scaffold composition are detailed in Table 3.
Figure 4. SEM images of tissue scaffolds a)10:0 (PVA: GEL) b) 10:1 (PVA: GEL) c) 10:3 (PVA: GEL) d) 10:1:1(PVA: GEL: Bioceramics).
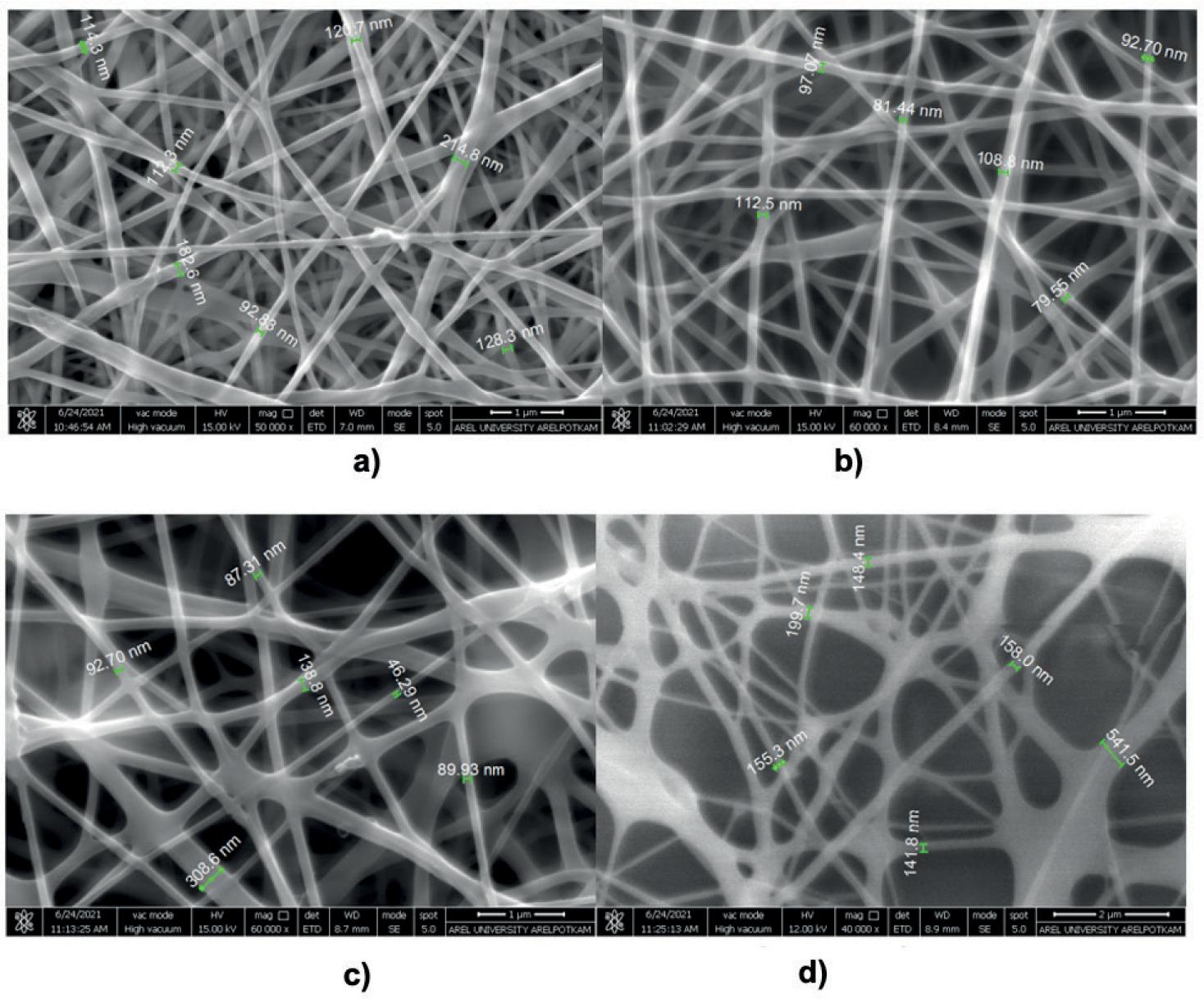
Table 3. Mean fiber diameters of tissue scaffolds.
| Sample | Mean Fiber Diameter (nm) |
|---|---|
| 10:0 (PVA: GEL) | 92-205 |
| 10:1 (PVA: GEL) | 72-142 |
| 10:3 (PVA: GEL) | 145-308 |
| 10:1:1 (PVA: GEL:Bioceramics) | 85-280 |
These findings underscore the critical role of additive materials in the composite, modulating the morphological properties of nanofiber scaffolds. Such insights are particularly valuable for advancing the design of scaffold materials in biomedical applications, where precise control over fiber dimensions is essential.
Mechanical characterization (Tensile test)
Tensile test results show that the 10:0 (PVA: GEL) sample exhibited a tensile strength of 6.25 MPa, which increased to 7.45 MPa with the addition of 1% GEL. The tensile strength in the 10:3 (PVA: GEL) sample was higher than that of the 10:1 (PVA: GEL) membrane. The gel is a protein-based polymer and has the capacity to form hydrogen bonds. PVA is also a polymer prone to hydrogen bonds. The increase in the gel ratio allows the formation of more hydrogen bonds in the PVA matrix. These bonds are thought to increase the mechanical strength of the nanofiber membrane by strengthening the interactions between the polymer chains (Qiao et al., 2015). Additionally, ncorporation of 1% bioceramic into the 10% PVA/1% GEL composite further enhanced the tensile strength of the 10:1:1(PVA: GEL: Bioceramics) scaffolds. The strongest sample in the study was identified as the 10:1:1(PVA: GEL: Bioceramics) composite, achieving a tensile strength value of 8.22 MPa (Table 4).
Table 4. Tensile strenghts of the composite scaffolds.
| Sample | Tensile Strength (MPa) |
|---|---|
| 10:0 (PVA: GEL) | 6.25 |
| 10:1 (PVA: GEL) | 7.45 |
| 10:3 (PVA: GEL) | 8.01 |
| 10:1:1(PVA:GEL:Bioceramics) | 8.22 |
As a result of the homogeneous wrapping of the PVA-GEL nanofibers by the bioceramics, nanofibers with larger surface areas were obtained. This is an indication of the linear increase observed in the tensile strength of the membranes. Moreover, the addition of bioceramic material contributed to the reinforcement of the polymer fibers by integrating into the matrix and enhancing the fiber strength (Hejazi et al., 2021; Şahin,2019; Altan et al., 2024).These findings highlight the synergistic effect of GEL and bioceramic additives in optimizing the mechanical properties of nanofiber scaffolds, making them suitable for applications requiring enhanced structural integrity.
Conclusions
This study successfully synthesized bioceramics from sea snail shells through the chemical precipitation method. Using PVA, GEL, and bioceramic powders, nanofiber-structured tissue scaffolds were fabricated via the electrospinning technique. The methodology employed demonstrated the effective integration of bioceramic components into the polymer matrix, producing a composite material with desirable properties for tissue engineering applications. The results of the study indicate that the synthesized bioceramics, when incorporated into tissue scaffolds, yield a biomaterial with promising characteristics, including ideal mechanical and structural properties. These scaffolds exhibited suitable porosity and nanofiber morphology, which are critical for mimicking the extracellular matrix and promoting cell adhesion, proliferation, and differentiation. The FTIR and SEM analyzes further confirmed the successful integration of bioceramics into the composite structure, demonstrating their compatibility with the polymer matrix and the maintenance of functional group integrity. The mechanical characterization results revealed that the composite scaffolds provide tensile strength, making them suitable for applications requiring load-bearing capacity, such as in bone and connective tissue regeneration. Moreover, the bioceramic component contributes to bioactivity and biocompatibility, facilitating interactions with surrounding tissues and promoting mineralization and osseointegration. Given these properties, it is anticipated that the composite scaffolds can serve as an interfacial layer in various tissue engineering applications. Their ability to create an environment conducive to cell adhesion and tissue regeneration suggests potential for use in dental applications, particularly for supporting bone and connective tissue formation. Additionally, the rapid biodegradability and bioresorbability of these scaffolds minimize long-term risks, making them a sustainable option for clinical applications. Future studies could focus on in vitro and in vivo assessments to further validate the bioactivity, biocompatibility, and mechanical performance of these scaffolds. Furthermore, exploring the scalability of the fabrication process and its adaptability to other bioceramic-polymer composites could pave the way for broader biomedical applications.