Introduction
Oral infections are types of infections that occur in and around the mouth. These diseases are usually caused by inadequate oral hygiene and the uncontrolled proliferation of pathogens (bacteria and fungi) (Li et al., 2000). Oral infections manifest as symptoms such as mouth sores, dental caries, and periodontal diseases, with dental caries being the most common (Nyvad & Takahashi, 2020). Dental caries occur when acids erode the enamel layer of the teeth, a process usually triggered by bacteria converting sugary and starchy foods in the mouth into acids These acids lead to the demineralization of tooth enamel, resulting in cavities (Sato et al., 2021). Streptococcus and Lactobacillus species are the two most prevalent bacteria that cause dental cavities (Caulifeld et al., 2015; Spatafora et al., 2024). Streptococci play a significant role in the development of dental caries, with Streptococcus mutans (S. mutans) being the most common and well-known causative agent (Lemos et al., 2019). S. mutans is a gram-positive bacterium that colonizes the natural oral microbiota. It can adhere to tooth surfaces and plaques, metabolize sugars into acids, and dissolve tooth enamel, causing cavities (Zhu et al., 2023). Additionally, S. mutans is a pathogenic organism that exhibits antibiotic resistance, posing a significant challenge in treating dental caries and other oral infections (Nomura et al., 2020; Zhang et al., 2022). Other Streptococcus species in the oral flora, such as Streptococcus mitis (S. mitis), are also associated with dental caries, though not as prominently as S. mutans (Bloch et al., 2024; Hohwy et al., 2001). S. mitis is a gram-positive bacterium, part of the normal oral microbiota, that adheres to tooth surfaces and contributes to plaque formation. However, its ability to ferment sugars and produce acids is not as strong as that of S. mutans, resulting in slower acid production and less harmful effects (Seow et al., 2009). Nevertheless, S. mitis is a common causative agent of endocarditis and is highly resistant to antibiotics (Ayi, 2007). Other bacterial species that can lead to dental caries include Lactobacillus species. Lactobacillus species are bacteria found in dairy products and the mouth. Due to their lactic acid fermentation, they cause tooth enamel erosion, leading to cavities (Wen et al., 2022). Specifically, Lactobacillus casei (L. casei), Lactobacillus fermentum (L. fermentum), and Lactobacillus acidophilus (L. acidophilus) are observed in dental caries. These bacteria can also cause nosocomial infections in immunocompromised individuals, and they exhibit antibiotic resistance, complicating treatment (Kullar et al., 2023).
β-lactam antibiotics are the most frequently prescribed broad-spectrum antibiotics, known for their minimal toxicity (Smith et al., 2018). The core structure of β-lactam antibiotics contains a four-membered saturated cyclic amide, known as the β-lactam ring, composed of one nitrogen and three carbon atoms (Fu et al., 2017). β-lactam group antibiotics were first discovered with the discovery of penicillin by Alexander Fleming in 1929. They were widely used to treat infectious diseases (Gaynes, 2017). These antibiotics are also recognized as biologically active molecules, exhibiting various activities such as antibacterial, antifungal, anti-inflammatory, and anticancer properties. The phenethylamine compound, a dopamine-like monoamine alkaloid, also possesses various biological activities (Yamase et al., 1996).
In this study, the antibacterial effects of previously synthesized phenethylamine-based β-lactam derivatives (7-12) (Figure 2) (Yildirim et al., 2022) on dental pathogens (S. mutans, S. mitis, L. casei, L. fermentum, L. acidophilus) were investigated. The antibacterial effect of the compounds used (1-12) on oral pathogens was reported for the first time in the literature in this study. In this respect, the study is quite original. This study contributes to the development of new approaches to the treatment of oral pathogens.
Figure 1. Imine derivatives (1-6), which are intermediate products used in the study.
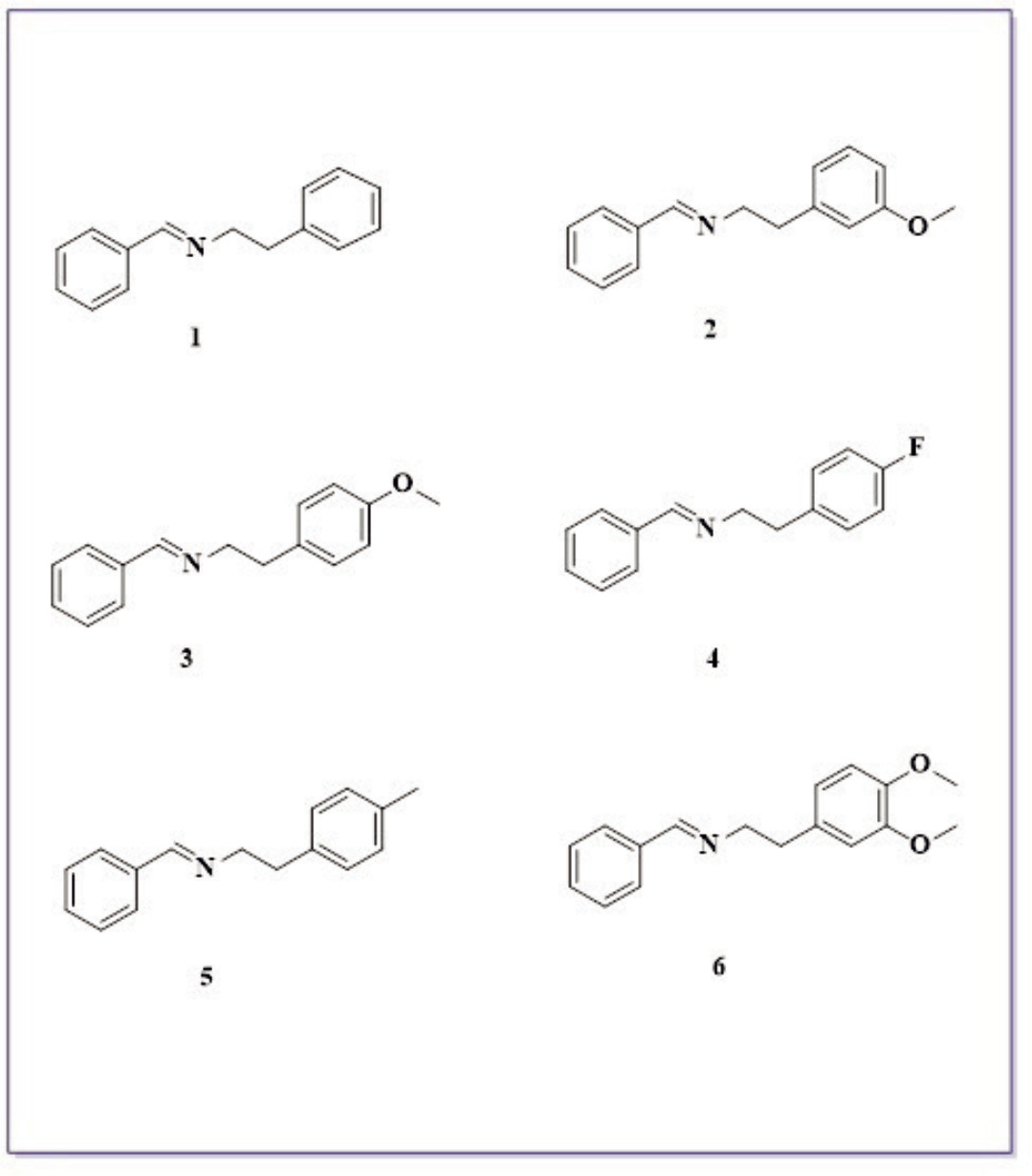
Figure 2. The target product used in the study was β-lactam derivatives (7-12).

Materials and methods
Materials
For the synthesis of β-lactam derivatives, the following chemicals were procured and used: Benzaldehyde (Sigma Aldrich), phenethylamine (Acros Organics), 3-methoxy phenethylamine (Alfa Aesar), 4-fluorine phenethylamine (J&K Scientific), 4-methyl phenethylamine (Acros Organics), 4-methoxy phenethylamine (J&K Scientific), acetoxy acetyl chloride (Acros Organics), ethyl acetate (TEKKİM), methylene chloride (Sigma Aldrich), molecular sieve 4Å (Sigma Aldrich), n-hexane (TEKKİM), sodium sulfate (MERCK), triethylamine (Sigma Aldrich), and chloroform D1 (Sigma Aldrich). For antibacterial studies, Luria Bertani Agar (LBA) (Miller MERCK), dimethyl sulfoxide (DMSO) (Merck), Mueller Hinton Agar (MHA) (Oxoid), Mueller Hinton Broth (MHB) (Biolife), and ampicillin were commercially purchased.
Synthesis design
In this study, β-lactam derivatives (7-12) and their imine intermediates (1-6) synthesized according to a previous report were used (Yildirim et al., 2022). Briefly, these compounds were synthesized as follows: Imines were obtained with a glass rod by adding substituted phenethylamines to benzaldehyde in the presence of a molecular sieve in a beaker. Then, it was dissolved in dichloromethane, and the molecular sieve was filtered on filter paper and removed. After the solution was evaporated, imine compounds (1-6) were obtained. The obtained imines were then reacted with acetoxy acetyl chloride in the presence of triethylamine and stirred overnight at room temperature. After the reaction, the mixture was extracted with water and dichloromethane. The desired compounds (7-12) were purified by column chromatography to obtain the pure product (Genc et al., 2016).
Antibacterial activity
In the biological assays conducted in the investigation, strains of S. mutans (ATCC 35668), S. mitis (NCIMB 13770), L. acidophilus (ATCC 4356), L. casei (ATCC 334), and L. fermentum (ATCC 9338) sourced from the repository of Erzurum Technical University Molecular Biology Laboratory were employed. The bacterial samples were cultivated in an LBA medium and utilized in investigations of antibacterial properties.
Agar well diffusion test
The agar well diffusion test is a widely used method in antibacterial tests. An agar well diffusion test was used to determine the antibacterial activity of imine and β-lactam derivatives. Bacterial inocula were prepared according to 0.5 McFarland standards and spread on the agar medium using a stick. Then, wells were created by piercing the agar with a cork borer. The wells were filled with compounds prepared at 200 µM. After 24 hours of incubation at 37°C, the diameters of the inhibition zones were measured to determine the antibacterial susceptibility of dental pathogens. To evaluate the activity of the synthesized compounds, Ampicillin at a concentration of 200 µM was used as a positive control and DMSO as a negative control. The diameters of the inhibition zones of the active compounds were measured as a result of the agar well diffusion test (Ozgeris, 2021).
Minimum inhibitory concentration (MIC)
Minimum inhibitory concentrations of compounds (7-12) that were effective in the agar well diffusion test were determined to determine the minimum antimicrobial concentration required to inhibit bacterial growth. This method uses a liquid medium to prepare serial dilutions ranging from 200 µM to 6.25 µM in a 96-well plate. Then, 100 µL of bacterial culture inoculum, adjusted to 0.5 McFarland standards, was added and incubated for 24 hours. After incubation, the lowest concentration at which no visible turbidity, indicating bacterial growth inhibition, was observed and recorded as the MIC value (Gormez et al., 2015).
Results and discussion
β-lactam antibiotics exhibit various biological activities and have garnered significant attention in bioorganic chemistry. Consequently, numerous methods for synthesizing β-lactam derivatives have been reported in the literature (Payili et al., 2018). Staudinger developed the first synthesis method for β-lactam derivatives, and many other methods have been introduced subsequently (Staudinger, 1907). The most common β-lactam synthesis approach involves the reaction of aromatic aldehydes with amines to form imines, which are then used to synthesize the corresponding β-lactam derivatives (Decuyper et al., 2018). This study successfully synthesized imine intermediates (1-6) from benzaldehyde and substituted phenethylamines using the given synthesis method. Subsequently, the target β-lactam derivatives (7-12) were obtained from the imines, with yields ranging from 48% to 91%. The synthesized compounds were evaluated for their antibacterial activities against pathogens responsible for oral infections.
The antibacterial activity of the synthesized compounds against oral pathogens was determined using the agar well diffusion method and the minimum inhibitory concentration (MIC) assay. The results of these studies are detailed in Table 1 and Table 2 below. According to the findings, while the target β-lactam derivatives exhibited antibacterial activity, the imine derivatives showed no antibacterial activity against oral pathogens.
Table 1. Agar well diffusion analysis results of novel compounds (1-12).
| Bacteria Strain | Zone Diameter of Compounds (cm) | Control | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Imine Derivatives (1-6) | β-lactam derivatives (7-12) | ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Ampicillin | |
| S. mutans (ATCC 35668) | No Zone | No zone | No zone | No zone | No zone | No zone | 2.1 | 1.5 | 1.9 | 1.7 | 2 | 0.9 | 2 |
| S. mitis (NCIMB 13770) | No zone | No zone | No zone | No zone | No zone | No zone | No zone | 1.5 | 1.1 | 1.6 | 1.9 | No zone | 1.3 |
| L. acidophilus (ATCC 4356) | No zone | No zone | No zone | No zone | No zone | No zone | 2.3 | 1.4 | 1.5 | 1.6 | 1.6 | No zone | 1.3 |
| L. casei (ATCC 334) | No zone | No zone | No zone | No zone | No zone | No z one | 1.7 | 1.6 | 1.6 | 1.7 | 1.8 | No zone | 1.2 |
| L. fermentum (ATCC 9338) | No zone | No zone | No zone | No zone | No zone | No zone | 1.9 | 1.4 | 1.5 | 1.5 | 1.6 | No zone | 1.3 |
Table 2. MIC values of effect compounds (7-12).
| Bacteria Strain | MIC value of β -lactam derivatives | ||||||
|---|---|---|---|---|---|---|---|
| 7 | 8 | 9 | 10 | 11 | 12 | Ampicillin | |
| S. mutans (ATCC 35668) | 25 μM | 25 μM | 25 μM | 25 μM | 25 μM | 12.5 μM | 6.25 μM |
| S. mitis (NCIMB 13770) | - | 50 μM | 50 μM | 50 μM | 100 μM | - | 50 μM |
| L. acidophilus (ATCC 4356) | 25 μM | 50 μM | 50 μM | 50 μM | 25 μM | - | 50 μM |
| L. casei (ATCC 334) | 25 μM | 25 μM | 50 μM | 50 μM | 50 μM | - | 100 μM |
| L. fermentum (ATCC 9338) | 12.5 μM | 25 μM | 12.5 μM | 25 μM | 12.5 μM | 12.5 μM | 50 μM |
In the literature, the antibacterial activity of imine derivatives against oral pathogens has been reported. For example, a study examined the antibacterial activities of imine derivatives against oral pathogens. As a result, it was reported that these compounds exhibited antibacterial activity (Sa’ad et al., 2022). In addition, another study included the synthesis of hemocompatible imine derivatives in which potent antimicrobial activity was observed (Altamimi et al., 2020). However, our study detected no antibacterial activity of imine derivatives against oral pathogens. This discrepancy may be attributed to the structural differences in the imine derivatives used in our study compared to those reported in the literature. It is well known that adding different side groups to the structure of organic compounds can significantly affect their biological activities. The imine compounds in our study have different side groups than those in the literature. This explains the lack of antibacterial activity of imine derivatives (1-6) against oral pathogens (Love & Ren, 1993).
The antibacterial activity of β-lactam derivatives against oral pathogens has garnered significant attention due to their effectiveness and the emergence of resistant strains. Recent studies highlight the potential of these compounds in combating oral infections, particularly those caused by resistant bacteria. Previous studies have investigated the effects of β-lactam derivatives on S. mutans. One study reported that β-lactam antibiotics are effective against antibiotic-resistant S. mutans, with a minimum inhibitory concentration (MIC) of 125 µM for aztreonam (Hirasawa & Takada, 2002). In our study, S. mutans was highly susceptible to compounds (7-12), exhibiting low MIC values and higher antibacterial activity than ampicillin. Compound 7 showed a significantly larger inhibition zone and a lower MIC value, indicating potent activity.
The increasing resistance of S. mitis to β-lactam antibiotics poses significant challenges in clinical settings. Research indicates that mutations in penicillin-binding proteins (pbp) are a primary mechanism behind this resistance, leading to reduced antibiotic affinity and efficacy (Nakayama et al., 2006). In the literature, S. mitis has been identified as harboring β-lactam resistance genes. Studies show that S. mitis strains exhibit multiple mutations in pbp genes, significantly lowering their affinity for ampicillin and cefaclor, resulting in high resistance levels. Clinical S. mitis strains have demonstrated MICs as high as 64 µg/ml for benzylpenicillin and 128 µg/ml for cefotaxime, indicating severe resistance (Wajima et al., 2022). In our study, compounds (8-11) displayed antibacterial activity against S. mitis, with inhibition zones larger than those produced by ampicillin. The MIC values were calculated to be between 50-100 µM, suggesting that these compounds are considerably potent compared to commercial products. Additionally, structural analysis revealed that electron-withdrawing groups at the meta and para positions of the compounds enhanced their antibacterial activity.
Lactobacillus strains, commonly found in dairy products, are known for their probiotic properties and ability to secrete natural antibiotics against pathogens (Mann et al., 2021). However, recent studies have reported the development of antibiotic resistance mechanisms in Lactobacillus strains (Chen et al., 2019). Notably, strains like L. casei have resisted β-lactam antibiotics. The blaTEM gene in 80% of these strains suggests potential resistance mechanisms against β-lactam derivatives. Additionally, L. casei may exhibit intrinsic resistance to certain β-lactams, potentially due to the presence of β-lactamases that can hydrolyze these antibiotics (Anisimova & Yarullina, 2019). This resistance poses a significant challenge, as β-lactam antibiotics used for treating oral pathogens may be inactivated by the β-lactamases produced by Lactobacillus (Anisimova et al., 2022). In our study, Lactobacillus strains exhibited potent activity against ampicillin, demonstrating their significant resistance capabilities. This finding highlights the need for ongoing research to better understand and address antibiotic resistance mechanisms in these probiotic strains, particularly in the context of oral infections.
Conclusions
In conclusion, in this study, substituted phenethylamine-based β-lactam derivatives (7-12) imine intermediates (1-6) were successfully synthesized in two steps. The antibacterial activities of these synthesized compounds against oral pathogens were studied and showed strong antibacterial activity. Based on these results, it is anticipated that β-lactam derivatives may serve as effective therapeutic agents against oral infections. Furthermore, the study highlights the potential of these β-lactam derivatives in biotechnological applications, especially in developing innovative antimicrobial agents to address the increasing challenge of antibiotic resistance and improve oral health solutions.