Introduction
Cancer and Alzheimer’s disease (AD) impose a significant socio-economic burden on society, resulting in nearly 10 million deaths and 19.3 million new cancer cases diagnosed in 2020 (Sung et al ., 2021). Additionally, at least 50 million people worldwide are living with AD or other forms of dementia (Breijyeh & Karaman, 2020). While treatment options for both conditions have expanded in recent years, they all exhibit significant drawbacks, including toxicity and resistance. Therefore, there is an urgent need for the development of novel and more effective agents to combat these diseases.
Breast cancer is among the most prevalent cancers affecting women globally. It develops when cells within the breast tissue multiply uncontrollably, leading to the formation of a tumor, which can become malignant (cancerous). Breast cancer can be classified into various subtypes based on the presence or absence of specific receptors on the surface of cancer cells. These receptors are critical for guiding treatment options and determining prognosis. Three primary types of receptors play key roles in breast cancer: Estrogen Receptor (ER), Progesterone Receptor (PR), Human Epidermal Growth Factor Receptor 2 (HER2). The estrogen receptor (ER) is a protein found in many breast cancer cells. Estrogen, a hormone primarily produced by the ovaries, binds to these receptors, promoting the growth and division of cancer cells. About 70-80% of breast cancers are estrogen receptor-positive (ER+), meaning they have ER proteins and respond to estrogen. ER+ breast cancers are often more responsive to hormone therapies because blocking estrogen from binding to these receptors can help slow or stop tumor growth (Weigel & Moore, 2010; Keenan et al., 2019).
The human estrogen receptor (ER) is a protein encoded by the ESR1 gene. It exists in ER-alpha (ERα) and ER-beta (ERβ) forms. ERα is more commonly implicated in breast cancer. When estrogen binds to the ERα receptor, it forms a complex that enters the cell nucleus and activates the transcription of genes that control cell proliferation, survival, and differentiation. The role of Estrogen in breast cancer development occurs via proliferation when Estrogen promotes cell division in breast tissue, and by overexposure to estrogen, which can lead to an increased risk of mutations, potentially resulting in cancer. If breast cancer is ER+, the cancer cells can use estrogen to grow and multiply, making it crucial to block estrogen’s effects in treatment, which then leads to tumor growth. For ER+ breast cancer, therapies focus on blocking estrogen’s ability to stimulate cancer growth. Common treatments targeting ER are based on selective Estrogen Receptor Modulators in which medications like Tamoxifen and Raloxifene block estrogen receptors in breast tissue, reducing the growth of ER+ tumors (Deal & Draper, 2006; Kumar & Kumar, 2018; Mason et al., 2020).
Recent research has investigated the complex relationship between cancer and Alzheimer’s disease (AD), with some studies suggesting a positive correlation, while others indicate an inverse relationship. Although cancer and AD often show an inverse relationship in terms of risk, they share several molecular mechanisms, and this complex interplay continues to be an area of active research. Several studies suggest that individuals with AD may have a reduced risk of developing certain types of cancer, such as colorectal or breast cancer. Similarly a higher risk of AD is associated with a lower likelihood of developing breast cancer. (Shafi, 2016; Yuan et al., 2024). Numerous studies indicate that cancer patients exhibit a reduced risk of developing Alzheimer’s disease (AD), and vice versa (Y. Li et al., 2024; R. Li et al., 2024; Wang et al., 2024). This inverse association is further reflected in the distinct pathological processes of both conditions: cancer is marked by uncontrolled cell proliferation, whereas AD involves progressive neuronal degeneration and cell death (Driver et al., 2012). An opposite finding reporting a positive correlation is based on selenium-containing compounds that were synthesized and evaluated for their antioxidant and antiproliferative activity in breast, lung, prostate, and colorectal cancer cell lines. Several of these molecules were found to exhibit dual activity as both anticancer and anticholinesterase agents (Kisla et al., 2024). In conclusion, the exact mechanisms behind this relationship are still unclear, but they may involve shared genetic pathways and environmental factors. These findings suggest that while AD and cancer may share some underlying biological mechanisms, their interaction is complex and more research is required to fully understand this relationship.
Recent work of our study group has primarily been focused on the discovery of natural source molecules for the treatment of Alzheimer’s Disease by targeting acetylcholinesterase and beta-site amyloid precursor protein-cleaving enzyme-1 (BACE-1) enzymes (Girgin et al., 2023; Girgin & Kantarci-Carsibasi, 2023). In the current study, a similar methodology will be employed to propose natural molecules as inhibitors of the ERα receptor protein through in silico virtual screening and molecular docking simulations. DrugBank (Wishart et al., 2006) and Zinc15 (Sterling & Irwin, 2015) databases are utilized to identify potential natural compounds with dual roles in treating both Alzheimer’s Disease and cancer. These results may subsequently be validated through in vitro biological assays and in vivo cancer models for future research.
Materials and methods
Protein preparation
The crystal structure of the ERα receptor protein (PDB ID: 1ERR), bound to Raloxifene, was retrieved from the Protein Data Bank. The preparation was conducted using Schrödinger’s Maestro Molecular Modeling Suite, utilizing the Protein Preparation Wizard module. The protein structure was refined during the process by correcting bond order and adding any missing hydrogen atoms. All heteroatoms, except the native ligand, were excluded, and water molecules within 5 Å of the binding site were retained. Missing loops or side chains were reconstructed using the Prime module. The protonation states were assigned using PROPKA at a pH of 7.0, and restrained minimization was performed with an RMSD threshold of 0.3 Å, applying the OPLS2005 force field for optimization (Jorgensen & Tirado-Rives, 1988).
Ligand preparation and virtual screening
Prior to docking simulations, the ligands were prepared with Schrödinger’s LigPrep module (Schrödinger, 2018; Madhavi-Sastry et al., 2013). Plausible 3D conformations are generated and correctly optimized. Ionization states, tautomers, and stereoisomers are assigned. Additionally, missing hydrogen atoms and bond orders are corrected. Energy minimization is performed to prepare the ligands suitable for molecular docking. Epik is used to generate the ionization states and tautomers at pH 7.0 ± 2.0 (Shelley et al., 2007), and chiralities present in the ligands’ 3D structures are used to obtain the stereoisomers. Natural source molecules, including metabolites (3093 molecules) and nutraceuticals (107 molecules), and also the FDA-approved drug subset (2619 molecules) were sourced from DrugBank separately. LigPrep was used to generate possible conformers from these molecules, making a total of 21.994 molecules which were subsequently docked into the ERα receptor protein binding site.
Molecular docking
Molecular docking was performed using the Glide SP (standard precision) algorithm in the Schrödinger Suite. A grid box was generated around the ERα receptor protein binding site, centered on the co-crystal ligand Raloxifene centroid, with a size chosen to accommodate ligands up to 20 Å in length. The same grid file was used throughout all docking simulations to ensure reliable comparisons. Ligands were kept flexible, and Epik state penalties were added to the docking scores. The docking protocol was validated by redocking the co-crystallized ligand Raloxifene, with an RMSD of 1.0 Å between the co-crystal and docked conformations. A total of 21.994 natural source molecules were directly docked, and molecules with high binding affinities were filtered and compared with the co-crystal drugs Raloxifene and also Tamoxifen, which are two commercially available drugs on the market.
Results and discussion
In this study, molecular docking simulations were performed to evaluate the binding affinity of various molecules to the ERα receptor protein (PDB ID: 1ERR). Docking simulations of nutraceutical molecules obtained from the DrugBank database revealed Galantamine, Thiamine, Queuine, and Folic Acid as the top four molecules with the highest docking scores. Docking simulations of metabolic molecules, on the other hand, identified metabolites of the control drugs Tamoxifen and Raloxifene, as well as several antidepressant drug metabolites that were deemed inappropriate. The FDA-approved drug subset of Drugbank yields Raloxifene, Astemizole (which is a withdrawn drug due to serious side effects), and Testosterone. Raloxifene is already the benchmark drug for treating breast cancer. By acting as an estrogen antagonist in breast tissue, Raloxifene lowers the risk of developing hormone receptor-positive breast cancer (Cummings et al., 1999). Testosterone is a hormone used to treat hypogonadism, breast carcinoma in women, hence already in use. Testosterone is reported to act as an anti-estrogen, particularly in hormone receptor-positive breast cancers. Competing with estrogen for binding to estrogen receptors, testosterone may inhibit estrogen’s stimulatory effects on tumor growth (Glaser & Dimitrakakis, 2015). Finally, the docking simulations of the Zinc15 library subset composed of biogenic and the FDA-approved drugs resulted in Astenile (Prasterone), Alogliptin, Paroxetine, and Galantamine. Astenile, which is a major C19 steroid produced by the adrenal cortex. It is also produced in small quantities in the testis and the ovary, and this molecule is already known as an estrogen receptor binder. Alogliptin is prescribed to manage high blood sugar levels in individuals with type 2 diabetes. Paroxetine, a selective serotonin reuptake inhibitor, is used to treat conditions such as major depressive disorder and panic disorder. Hence, these molecules are not preferred to treat breast cancer ERα. As the literature evaluations, docking scores and safety considerations are taken into the picture, the molecules: Galantamine, Thiamine, Queuine, and Folic acid come forward. These molecules are mentioned to be involved in various cancer types before as well as AD. Hence, they may have a dual role in both diseases. Galantamine is a natural alkaloid primarily used to treat mild to moderate Alzheimer’s disease. Originally, it was extracted from the bulbs and flowers of plants but can also be synthesized (Tariot et al., 2000). Queuine is a naturally occurring biochemical compound present endogenously in the human body. It plays a critical role in the synthesis of essential chemicals such as tyrosine, serotonin, dopamine, epinephrine, norepinephrine, nitric oxide, and various lipids, contributing to numerous physiological processes (Fergus et al., 2015). Thiamine, commonly known as vitamin B1, is essential for intracellular glucose metabolism and supports the normal function of vital systems, including the cardiovascular, nervous, and digestive systems, in most organisms. It is regarded as one of the key vitamins necessary for maintaining overall health (Tylicki et al., 2018). Folic acid is the synthetic form of folate (vitamin B9) that is essential for numerous biological processes. It plays a crucial role in DNA synthesis, repair, and methylation, as well as in the production of red blood cells (Bailey, 2009). Figure 1 demonstrates the molecular structures of these molecules together with control-approved drugs.
Figure 1. The chemical structures of three promising natural molecule candidates proposed for ERα receptor inhibition along with the FDA-approved drugs Raloxifene and Tamoxifen.
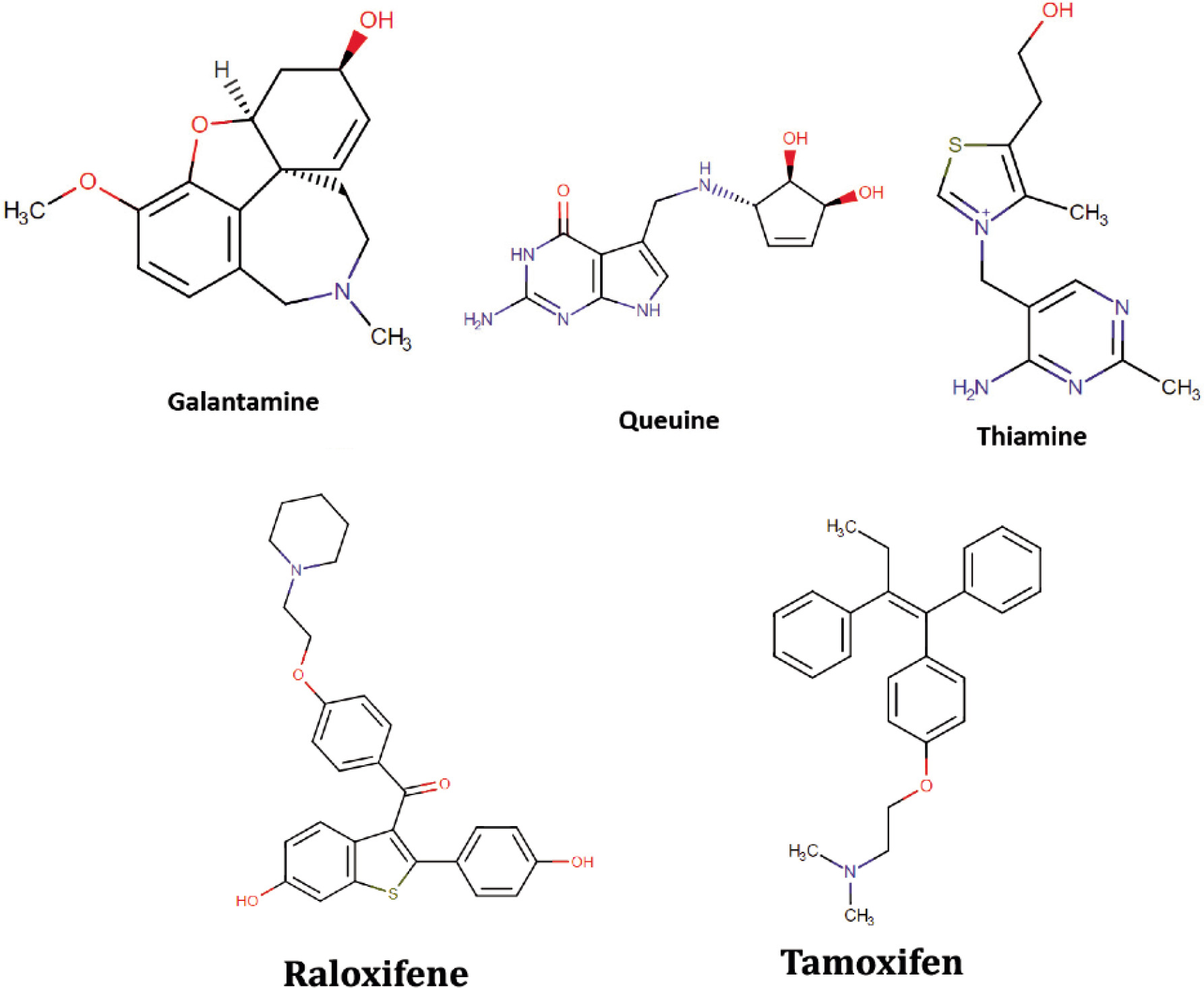
Figure 2 illustrates the virtual screening protocol conducted over the DrugBank and Zinc15 libraries to identify potential inhibitors of the ERα receptor protein. Subsets were downloaded from DrugBank, specifically the nutraceuticals, metabolites, and the FDA-approved molecule sets. Additionally, one subset from the Zinc15 library, consisting of the FDA-approved and biogenic molecules, was obtained. Consequently, four molecule sets underwent virtual screening, beginning with the LigPrep module to generate possible conformers. As shown in Figure 2, these subsets were docked directly into the ERα receptor binding site using Glide SP and ranked based on their docking scores, from the strongest binder to the weakest. Promising compounds identified from each set are also displayed. The green ticks indicate molecules not previously recognized as ERα receptor protein inhibitors, suggesting the potential for further investigation due to their properties. In contrast, the red crosses mark molecules that are either already in use as ERα receptor inhibitors for breast cancer treatment, withdrawn for various reasons, deemed unsafe, or considered irrelevant and not essential for further studies as discussed above in detail.
Figure 2. Schematic flow for the filtering procedure conducted on Drug Bank and Zinc Databases targeting ERα receptor protein.
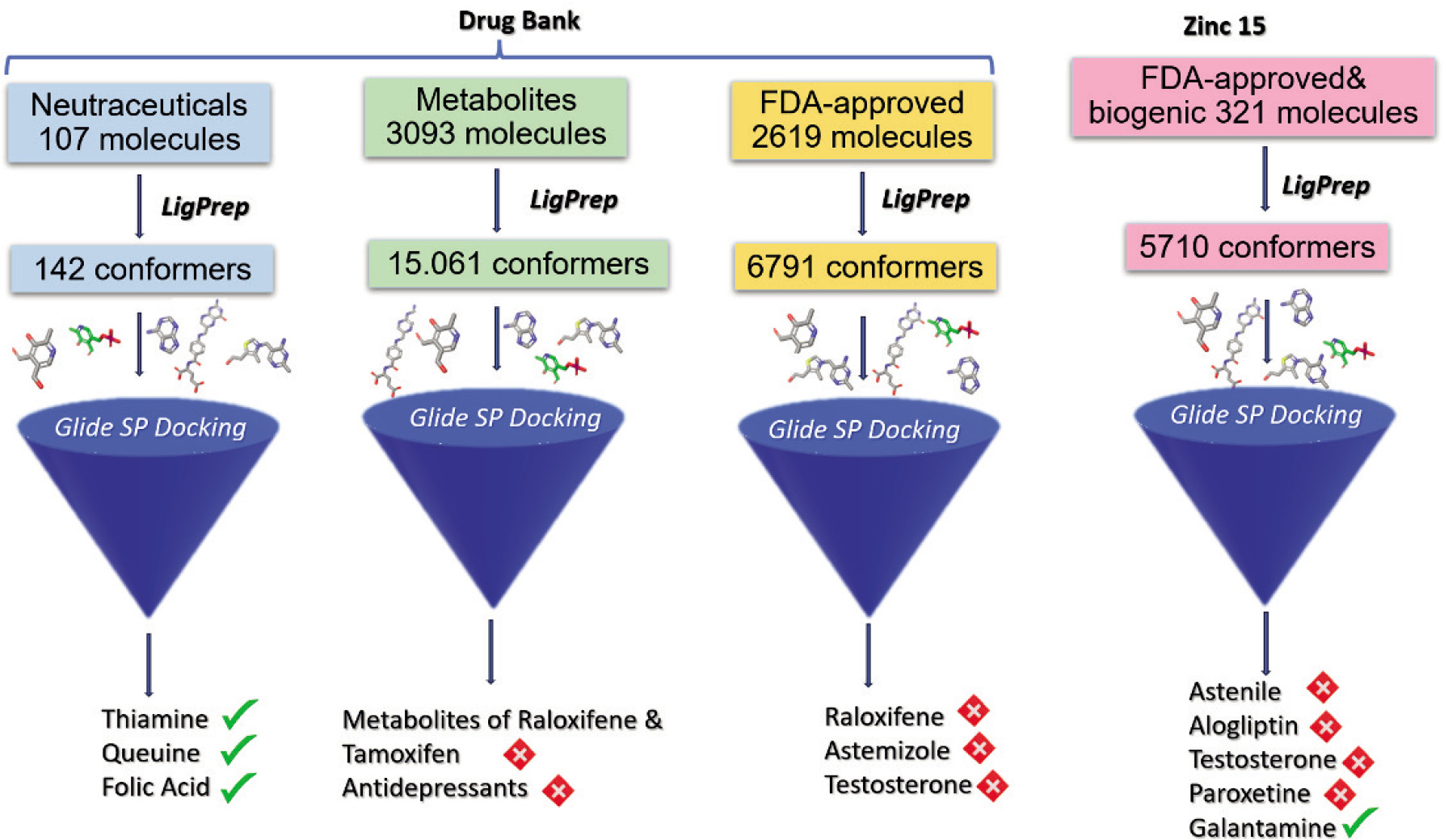
Table 1 presents the properties of the natural hit molecules, alongside the reference control drugs Raloxifene and Tamoxifen. This table details the associated Drug Bank IDs, the origins of each molecule, their relevance to specific cancer types, and the corresponding references. From the table, it can be inferred that Thiamine, Folic acid and Galantamine have been previously linked to breast cancer. However, to the best of our knowledge, literature does not report a connection between Queuine and breast cancer. Nonetheless, all four leading natural molecules have been noted concerning various cancer treatments.
Table 1. Properties of lead natural molecules identified as potential ERα receptor protein inhibitors
| Molecule name | DrugBank ID | Origin | linked to cancer before? | Reference |
|---|---|---|---|---|
| Thiamine | DB00152 | nutraceutical | breast, colon, pancreatic, and hematological cancers | Liu et al., 2018; Comín-Anduix et al., 2001; Bruce et al., 2003, Iimura et al., 2021 |
| Queuine | DB14732 | nutraceutical | colon, ovarian, brain, lung, leukemia, and lymphomas | Baranowski et al., 1994; Aytac & Gündüz, 1994; Huang et al., 1992; Fergus et al., 2015 |
| Folic acid | DB00158 | nutraceutical | colorectal, breast, pancreatic, and cervical cancers | Qin et al.,2013 |
| Galantamine | DB00674 | botanical FDA-approved | colorectal, and breast | İnce et al., 2023 |
| Tamoxifen | DB00675 | synthetic FDA-approved control drug | FDA-approved breast cancer drug (selective estrogen receptor modulators) | Jordan, 2003; Jordan, 2006 |
| Raloxifene | DB00481 | synthetic FDA-approved control drug | FDA-approved breast cancer drug (selective estrogen receptor modulators) | Deal & Draper, 2006 |
Our recent study on Alzheimer’s disease and acetylcholinesterase enzyme inhibition highlighted the effects of Thiamine and Queuine. The efficacy of these compounds was compared to that of Galantamine, a benchmark natural drug for Alzheimer’s treatment. Results demonstrated that Queuine exhibited comparable docking scores, binding affinity, and a more potent IC50 value than Galantamine. Conversely, Thiamine was found to be non-cytotoxic even at high concentrations (Girgin et al., 2023). Folic acid has also been recognized for its effects on both various cancer types and Alzheimer’s disease. Therefore, we emphasize Queuine, as it has not been previously associated with breast cancer. Additionally, all four molecules listed in Table 1 have been reported to play a role in both cancer and Alzheimer’s disease, suggesting a potential positive correlation for molecules that may have dual therapeutic roles in these conditions.
Figure 3 depicts the ERα receptor protein in complex with the FDA-approved drug Raloxifene, which is also the co-crystal ligand in Protein Data Bank structure pdb ID: 1ERR. The figure shows both 3D and 2D docked conformations of Raloxifene and interactions it accomplishes with the binding site. The hydrogen bonding with Glu 353 and Arg 394, and pi-pi stacking through Phe 404 are the most significant interactions.
Figure 3. Raloxifene in complex with Erα receptor protein: 3D and 2D interactions in the active site.
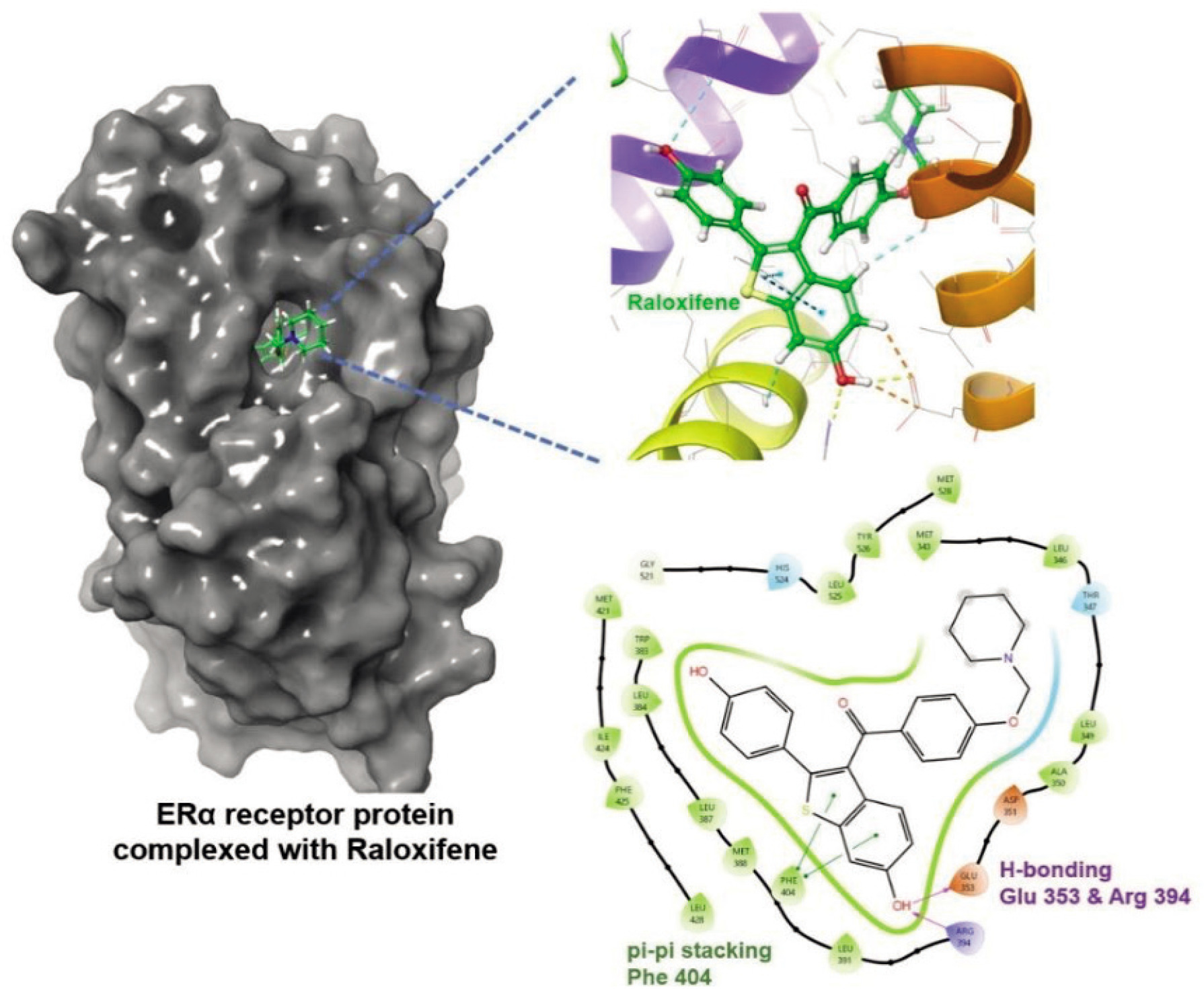
Figure 4 depicts Queuine, Thiamine, Galantamine and Folic acid in complex with target receptor ERα. Interactions are displayed for Queuine (Fig 4a and 4b), Thiamine (Fig 4c and 4d), and Galantamine (Fig 4e and 4f) as 3D and 2D representations. Queuine forms hydrogen bonds with Glu 353 and Thr 347, achieving a docking score of -8 kcal/mol. Thiamine interacts via π-π stacking with Phe 404, resulting in a docking score of -8.3 kcal/mol. Galantamine, like Queuine, forms hydrogen bonds with Glu 353, with a docking score of -8.8 kcal/mol. Folic acid performs hydrogen bonds through Glu 353, Leu 387 and Tyr 526, in addition to pi-pi interaction conducted with Phe 404, all contributing to a docking score of -7.5 kcal/mol. The control drug Raloxifene shows interactions with Glu 353 and Arg 394 through hydrogen bonding, along with π-π stacking with Phe 404, and yields a superior docking score of -11.4 kcal/mol. The presence of hydrogen bonding with the Glu 353 residue appears to be a critical factor, as it is consistently observed among the top candidate molecules. The details of the residue interactions and interaction types are also tabulated in Table 2.
Figure 4. Queuine, Thiamine, Galantamine and Folic acid in complex with target receptor ERα: 3D and 2D interactions.
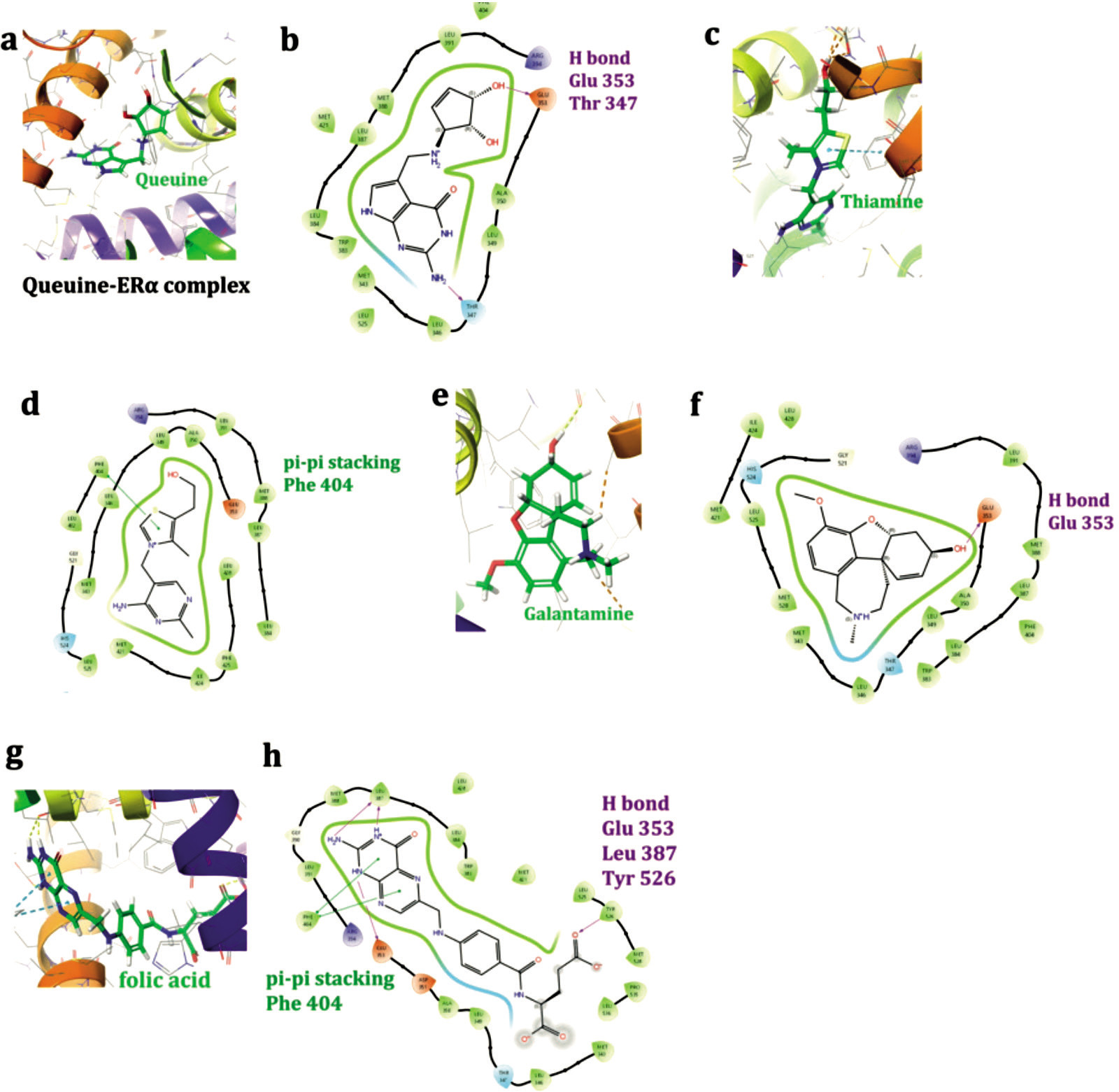
Table 2. Results of docking simulations: docking scores, interacting residues, and interaction types of proposed compounds compared with approved drugs.
| Molecule | ERá receptor DScore(kcal/mol) | Interacting residues | Interaction type |
|---|---|---|---|
| Galantamine | -8.8 | Glu 353 | H-bond |
| Thiamine | -8.3 | Phe 404 | π- πstacking |
| Queuine | -8 | Glu 353 Thr 347 | H-bond H-bond |
| Folic acid | -7.5 | Glu 353 Leu 387 Tyr 526 Phe 404 | H-bond H-bond H-bond π - π stacking |
| Tamoxifen | -9.6 | Asp 351 Asp 351 | H-bond Salt bridge |
| Glu 353 | H-bond | ||
| Raloxifene | -11.4 | Arg 394 Phe 404 | H-bond π-π stacking |
Table 3 presents the drug-like properties of the proposed natural compounds alongside those of the control drugs. Compared to the FDA-approved drugs Raloxifene and Tamoxifen, the natural molecules Queuine, Galantamine, Thiamine, and Folic acid exhibit significantly lower lipophilicity values. This suggests they are more water-soluble and hydrophilic, which may contribute to better bioavailability and distribution in aqueous environments. Additionally, all molecules passed the AMES test for mutagenic potential, indicating no toxicity or mutagenicity concerns.
Table 3. Predicted druglike and ADMET properties of proposed lead natural molecules compared to Raloxifene and Tamoxifen.
| Molecule | MW1 (g/mol) | log P2 | HBD3 | HBA4 | TPSA5 (Å) | HIA6 (%) | AMES7 toxicity |
|---|---|---|---|---|---|---|---|
| Queuine | 277.3 | -1.6 | 7 | 7 | 136.5 | 94.6 | Non-toxic |
| Galantamine | 287.4 | 1.2 | 1 | 4 | 41.9 | 99.9 | Non-toxic |
| Thiamine | 265.4 | -2.1 | 2 | 4 | 75.9 | 79.7 | Non-toxic |
| Folic Acid | 441.4 | -0.5 | 6 | 12 | 208.9 | 79.5 | Non-toxic |
| Raloxifene | 473.6 | 5.5 | 2 | 5 | 70 | 98.6 | Non-toxic |
| Tamoxifen | 371.5 | 5.9 | 0 | 2 | 12.5 | 99.7 | Non-toxic |
1Molecular Weight (from Pubchem, Kim et al., 2023)
2octanol/water partition coefficient (from ALOGPS, Tetko et al., 2005)
3Hydrogen bond donor (from Drug Bank, Daina, et. al., 2017; Wishart et al., 2006)
4Hydrogen bond acceptor (from Drug Bank, Wishart et al., 2006)
5Topological polar surface area (from Drug Bank, Wishart et al., 2006)
6Human intestinal absorption from (admetSAR, Cheng et al., 2012)
7Mutagenic potential (from admetSAR Cheng et al., 2012)
Conclusion
This study focuses on identifying new natural compounds to inhibit Estrogen Receptor Alpha (ERα) for the treatment of breast cancer. Although the FDA-approved drugs Raloxifene and Tamoxifen are effective in managing breast cancer, their use is often accompanied by side effects. The compounds proposed in this research aim to deliver comparable efficacy with reduced toxicity compared to these FDA-approved drugs. Through in silico virtual screening of extensive drug databases, Drug Bank and Zinc15, followed by molecular docking simulations, several promising molecules were identified. In particular, molecules such as Queuine, Thiamine, Galantamine, and Folic Acid exhibited strong binding affinity to Estrogen Receptor Alpha and demonstrated promising effects against Alzheimer’s disease, opening doors for a dual-purpose therapeutic approach. It is important to note that molecular docking simulations have certain limitations. The docking simulations employed in this study considered the target protein as a rigid structure, whereas proteins undergo dynamic conformational changes in real biological systems. Therefore, the obtained results may not fully reflect the interactions occurring in a more realistic environment where the protein is flexible and surrounded by water molecules. The effect of water is often not explicitly modeled or is modeled using simplified water models in virtual docking methods. This can lead to results that do not accurately represent the interactions in a real biological system. Water molecules play a significant role in protein-ligand interactions and can influence interactions such as hydrogen bonding. Therefore, in addition to molecular docking simulations, conducting molecular dynamics simulations would be crucial for gaining a deeper understanding of the dynamic interactions of these compounds with the protein and elucidating the binding mechanisms. For future work conducting molecular dynamics simulations can provide more in-depth information about the selectivity and side effects of these compounds, thus contributing to the identification of more reliable candidate molecules. The Log P values of the nutraceutical molecules are lower than those of the control drugs, indicating their hydrophilic nature. While this may offer an advantage in terms of oral bioavailability, it could pose a disadvantage to cell membrane penetration and interaction with the binding site, which favors more hydrophobic characteristics. These challenges can be addressed by employing appropriate drug delivery systems, such as formulating the compounds with more hydrophobic encapsulations to enhance binding affinity and cellular uptake. The findings of the present study constitute a significant step toward developing novel therapeutic strategies for both cancer and neurodegenerative diseases. However , in vivo efficacy and safety profiles of these compounds need to be further supported by more extensive studies.