Introduction
Trigeminal neuralgia is a type of short-term pain that develops suddenly and very severely, upon stimulation of the 5th cranial nerve in the face. It is popularly classified as one of the most unbearable pains. It causes severe pain in the face and if untreated, it is a gravedisease that disrupts the patient’s quality of life and social activity. For this reason, it is also known as suicidal illness (Merskey & Bogduk, 1994). Trigeminal neuralgia often manifests with dental pain, leading to treatments such as tooth extraction or root canal procedures. However, these treatments may not effectively alleviate the pain, as the source of the pain lies within the trigeminal nerve branches extending to the tooth’s root. In some cases, the pain originates from the trigeminal nerve itself or even from the exit region of the brain stem (Zakrzewska, 2002). Therefore, conventional dental interventions may not address the underlying cause of trigeminal neuralgia.
The methylation of catechol fragments is an essential process carried out by the enzyme CatecholO-methyltransferase (COMT), which serves to deactivate catechol hormones, neurotransmitters, and foreign catecholamines found within the body (Axelrod & Tomchick, 1958). In addition to its role in catecholamine regulation, COMT is also implicated in melanin biosynthesis (Pavel, 1993). Moreover, researchers have proposed associations between COMT and various mental disorders, including depression and schizophrenia (Murphy & Wyatt, 1975). The amino acid valine at codon 108/158 results in a heat-stable version of COMT with high activity, whereas methionine at the same position produces a heat-sensitive form of COMT with lower activity. This functional variation in the COMT gene leads to alterations in the activity of the COMT enzyme. Accordingly, the gene is thought to play a role in the pathogenesis of neuropsychiatric disorders, schizophrenia, migraine, Parkinson’s disease and bipolar affective disorders (Daniels et al., 1996; Emin et al., 2001).
Recent research has suggested that various variants of the COMT gene are associated with cranial pain disorders (Meloto et al., 2015). Polymorphisms in the COMT gene have been associated with a variety of pain conditions, including fibromyalgia (Gürsoy et al., 2003), temporomandibular joint disorder (Brancher et al., 2021), migraine (Emin et al., 2001), irritable bowel syndrome (Karling et al., 2001) and ongoing surgical pain (Dharaniprasad et al., 2000). Anotherstudy has shown that the incidence of the disease in patients with trigeminal neuralgia is positively associated with genetic polymorphisms, such as familial inheritance (Panchagnula et al., 2019). Understanding the causes and providing treatment for trigeminal neuralgia has been one of theextensively studied fields With the increase in molecular genetic studies on trigeminal neuralgia, where early diagnosis and treatment are important, it is predicted that the causes of the disease will be better understood, and more permanent genetic solutions can be provided.
The aim of this study is to investigate the hypothesis that rs4680 (Val158Met) polymorphism of the COMT gene may cause trigeminal neuralgia disease, which has been supported by various molecular studies.
Materials and methods
Study group
The research encompassed 10 patients diagnosed with trigeminal neuralgia who sought treatment at the Department of Oral, Dental, and Maxillofacial Surgery at Marmara University, Faculty of Dentistry. 30 healthy individuals voluntarily participated in the study as control group. The research protocol was developed following the 2015 guidelines of the Helsinki Declaration and received approval from the Clinical Research Ethics Committee of Marmara University’s Faculty of Medicine (protocol code: 09.2021.324). Prior to the study, every participant signed consent forms that contained comprehensive details about the study’s protocol, findings, and assessment of those findings.
Inclusion criteria for the study:
-
Trigeminal neuralgia that has been diagnosed by a neurologist
-
Episodic attacks of trigeminal neuralgia felt in the maxilla or mandible
-
Unilateral neuralgia in the distribution of the second and/or third branches of the trigeminal nerve
-
No presence of genetic disorders in their families or in themselves.
-
Participants’ age between 18-65.
Exclusion criteria for the study:
-
The existence of organic elements like tumors or various brain lesions, such asmultiple sclerosis.
-
The presence of unusual facial pain, with symptoms that resemble trigeminal neuralgia.
-
Presence of a family history of genetic disease.
-
Individuals outside the age range of the study
DNA isolation and genotyping
Peripheral blood samples obtained from patient and control samples were used to isolate DNA (Kazancı et al., 2021). DNA isolation was conducted using the PureLink DNA isolation kit (Invitrogen, Carlsbad, CA, USA) Isolated DNA samples were stored at -20°C until gene region analysis.
Real time- PCR analyses
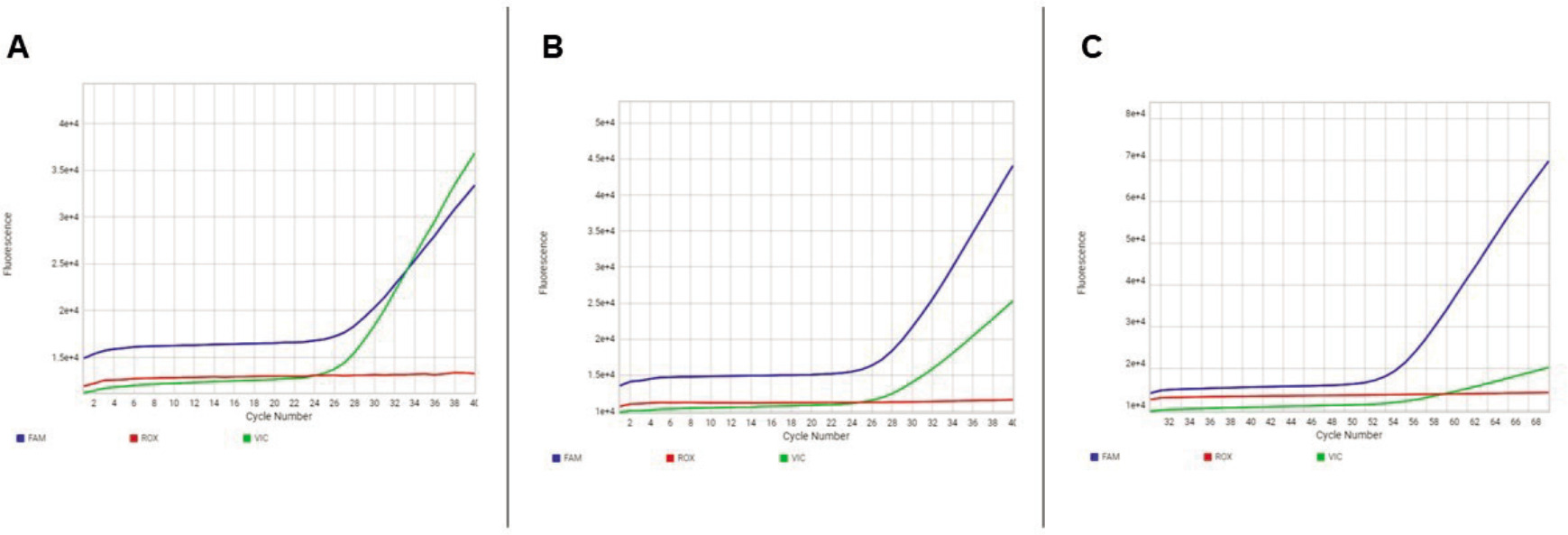
Real-Time PCR was used for the genotyping of the COMT rs4680 polymorphism, using StepOnePlus device, utilizing Taqman SNP Genotyping Assays kits in accordance with the protocols provided by the manufacturers. A and G alleles were determined using Taqman VIC and FAM primers, respectively (Figure 1).
Figure 1. Real-Time PCR image of COMT rs4680 polymorphism. FAM represents the G allele (blue curve), whereas VIC signifies the A allele (green curve). The (A) single green curve represents the homozygous genotype AA, (B) the combination of green and blue curves signifies the heterozygous genotype AG, while (C) the single blue curve reflects the homozygous genotype GG.
Statistical analysis
Statistical analyses were conducted using the SPSS 25.0 software and the χ2 (chi-square) test. A p value of less than 0.05 was regarded as statistically significant.
Results and discussion
COMT rs4680 polymorphism analysis revealed that 2 out (20%) of 10 trigeminal neuralgia patients had AA genotype, 6 (60%) had AG genotype and 2 (20%) had GG genotype. When analyzing the distribution of alleles, it was found that 50% were allele A and 50% were allele G. In the control group (n=30), 5 patients had the AA genotype, 14 patients had the AG genotype, and 11 patients had the GG genotype. When analyzing the distribution of alleles, it was found that 40% were allele A and 60% were allele G. The distributions of genotypes and alleles in the patient and control groups were summarized in Table 1. At the same time, a statistical analysis was performed using the chi-square test to compare the distribution of genotypes and alleles of the COMT rs4680 polymorphism in the patient and control groups. The obtained p-values indicate that there is no statistically significan difference between the frequency of the genotype (p=0.6202) and the allele (p=0.4334). These results indicate that there is no significant genetic difference between patients with trigeminal neuralgia and healthy controls regarding the COMT rs4680 polymorphism.
Table 1. Genotypic and allelic distribution of COMT rs4680 polymorphism in patients with trigeminal neuralgia and control group.
| Genotype | Allelic Distribution | ||||||
|---|---|---|---|---|---|---|---|
| AA | AG | GG | p Value | A | G | p Value | |
| TN (n=10) | 2 | 6 | 2 | 10 | 10 | ||
| Percentage | 20% | 60% | 20% | 50% | 50% | ||
| Control (n=30) | 5 | 14 | 11 | 0,6202 | 24 | 36 | 0,4334 |
| Percentage | 16% | 47% | 37% | 40% | 60% | ||
Significance was evaluated at a minimum level of p<0.05. The χ2 test was utilized to compare the results with the control group.
COMT, encoded by a gene located on chromosome 22q11.2, catalyzes the degradation of catecholamines, particularly dopamine. It has been shown that a functional polymorphism in the 158th codon (Val158Met) of this gene, which involves substitution of methionine for valine, causes a 4-fold variation in enzyme activity (Taerk et al., 2004).
Several genetic studies have investigated the underlying causes of trigeminal neuralgia (TN) and identified potential genes associated with the condition. Costa et al. (2019) examined polymorphisms in the SCN9A gene, which encodes the NaV1.7 sodium channel, and the NTRK1 gene, which encodes the TrkA receptor. Although they found no direct association between these polymorphisms and NTN, they suggested that other genotypes may still play a role in disease pathogenesis. Similarly, Tanaka et al. (2016) discovered the Met136Val mutation in the SCN8A gene encoding the sodium channel NaV1.6, which may increase the excitability of trigeminal ganglion neurons and contribute to TN.
Siqueira et al. (2009) observed downregulation of the sodium channel NaV1.7 and upregulation of NaV1.3 in patients with TN, suggesting that TN may be a type of channelopathy, a condition caused by dysfunction of ion channels. Further studies by Gambeta et al. (2021) showed that mutations in CACNA genes encoding calcium channels can increase neuronal excitability and potentially increase susceptibility to TN. Cui et al. (2014) found that a polymorphism in the SLC6A4 gene encoding the serotonin transporter (5-HTT) was associated with higher susceptibility to TN, increased pain severity, and better response to carbamazepine (CBZ) treatment. This suggests that serotonin transporter genotype may play a crucial role in modulating TN pain and treatment outcomes, as TN patients have a higher prevalence of this polymorphism compared to the general population.
A meta-analysis conducted by Barbosa et al. (2012) has revealed that individuals carrying the Met allele of the rs4680 polymorphism in the COMT gene exhibit a heightened susceptibility to fibromyalgia or widespread chronic pain. This association was evident across various Caucasian populations, as well as among Israelis and Turks. Furthermore, the study suggests a similar correlation between fibromyalgia and pain sensitivity with the rs4680 polymorphism in the Brazilian Caucasian population, which comprises a blend of Hispanic Caucasians, indigenous Brazilians, and individuals of African descent. Additionally, several studies propose that the rs4680 polymorphism may play a regulatory role in conditions linked to fibromyalgia pain (Finan et al., 2010, 2011).
While the COMT rs4680 polymorphism may not be directly linked to migraine headache, it could still play a role in influencing the risk of other chronic headaches or even impact the migraine phenotype. Hagen et al. (2006) proposed that the Met allele might serve as a risk factor for headaches other than migraine among Norwegian women. Similarly, Park et al. (2007) demonstrated that Korean female migraine patients carrying the Met allele experienced more severe headaches, along with symptoms like nausea and vomiting. Considering COMT’s involvement in estrogen metabolism and the role of estrogen in migraine pathophysiology, COMT polymorphisms have been posited to predispose individuals to migraine. Although a meta-analysis examining the association between the COMT gene and musculoskeletal disorders found no significant link between the rs4680 polymorphism and musculoskeletal pain, it did reveal that the COMT rs4633 polymorphism may regulate the recovery of disability index scores after surgery (Dai et al., 2010). Moreover, COMT pain sensitivity haplotypes have been shown to impact pain ratings, including catastrophic pain ratings, suggesting a potential modulatory role for COMT in chronic pain (George et al., 2008).
Research exploring the link between COMT and fibromyalgia has primarily centered on a functional polymorphism that results in a single substitution of methionine for valine in exon 4 (Lachman et al., 1996). This amino acid sequence alteration affects the activity of the COMT enzyme, with homozygosity for the valine allele exhibiting 3-4 times higher activity compared to the methionine allele. Studies have demonstrated that lower activity associated with homozygosity for the methionine allele leads to significantly reduced dopamine levels in postsynaptic neurons (Egan et al., 2001). In temporomandibular disorder (TMD), chronic and persistent pain states have been found to induce notable changes in catecholamine physiology, which are closely linked to COMT enzyme activity (Nackley et al., 2007). The majority of genetic association studies related to COMT have concentrated on the commonly studied rs4680 SNP. The A allele of the rs4680 polymorphism has been linked to a higher likelihood of experiencing postoperative pain (Ahlers et al., 2013), fibromyalgia (Cohen et al., 2009), and arthritis (Van Meurs et al., 2009). Furthermore, the rs4680 polymorphism has been linked to important intermediate phenotypes, including experimental pain (Zubieta et al., 2003), anxiety (Fernandez-de-Las-Penas et al., 2012), depression, and attention (Voelker et al., 2009).
Trigeminal neuralgia (TN) presents as a severe facial pain disorder characterized by an elusive etiology and uncertain genetic underpinnings. Unfortunately, TN often goes undiagnosed or misdiagnosed, contributing to its challenges in clinical management. Research indicates that the incidence of TN varies, with reported rates ranging from 4.3 to 27 new cases per 100,000 individuals annually. Moreover, TN is more commonly observed in women and tends to escalate with advancing age. Community-based studies estimate the lifetime prevalence of TN to be approximately 0.16-0.3%.
The typical onset age for classical TN is around 53 years, while secondary TN manifests earlier, around 43 years, although onset timing can vary considerably across age groups. Notably, secondary TN accounts for 14-20% of patients in tertiary care settings (Mannerak et al., 2021). A Brazilian study showed that there was no significant difference in the genotype distribution of COMT rs4680 polymorphism analyzed between TN patients and controls (Romero et al., 2021). This study compared the genotypic and allelic distribution of COMT rs4680 polymorphism between patients with trigeminal neuralgia and healthy controls. In our study, AG genotype was found to be more common (60%) in patients with trigeminal neuralgia and it was hypothesized that this genotype may affect susceptibility to the disease. However, the results of the chi-square test showed that this difference was not statistically significant (p=0.6202), suggesting that genotype distribution does not have a strong association with trigeminal neuralgia. There was also no statistically significant difference in allele distribution between A and G allele frequencies (p=0.4334).
Conclusions
These data suggest that COMT rs4680 polymorphism may not play a critical role in the pathogenesis of trigeminal neuralgia. However, due to the limited sample size of our study, these results need to be confirmed by studies with larger sample groups. Barbosa et al. (2012) found that COMT rs4680 polymorphism may have a significant association with other pain disorders, especially fibromyalgia and temporomandibular joint disorders. Therefore, larger and comprehensive genetic studies are needed to better understand the role of this polymorphism in neuropathic pain syndromes such as trigeminal neuralgia. Although the results of our study do not fully reveal the potential impact of COMT rs4680 polymorphism on the development of trigeminal nerve diseases. Patients with neuralgia emphasize the importance of genetic research in this field. In particular, a better understanding of the genetic factors involved in the pathogenesis of neuropathic pain syndromes may facilitate the development of more personalized treatment approaches in the future. In this context, examining larger patient groups and evaluating different genetic variants together in future studies may help to better understand the genetic basis of trigeminal neuralgia and other chronic pain disorders.