Introduction
Carbon cloth (CC), which is a conductive textile, has drawn attention due to its outstanding properties, such as good electrical conductivity, stability, high surface area, high capacitance (Bi et al., 2016; Shao et al., 2017). CC-based electrodes (CCE) are advantageous, because they are inexpensive, portable, disposable, and flexible. Since CC has a porous structure, it offers fast ion transport through increased number of channels, leading to improved diffusion of electrolyte into the electrode (Razali & Majid, 2019). In addition, CCE has a simple operation compared to the commercial glassy carbon electrode (GCE), since pretreatment steps such as polishing and ultrasonic cleaning are not necessary before using CCE. CC substrate is more advantageous over glass-based or metal-based electrodes due to its thin and soft nature, and ease of obtaining various shapes using scissors. Thus, CCEs have been widely used in various applications, such as double-layer capacitors (Galinski & Stepniak, 2009), super capacitors (Lewandowski, Olejniczak, Galinski, & Stepniak, 2010), microbial fuel cells (Tsai, Wu, Lee, & Shih, 2009) and wastewater treatment (Huang & Su, 2010).
Afkhami et al. modified the carbon cloth using distilled water and acid, and successfully applied it for removal of nitrate and nitrite from water samples at neutral pH (Afkhami, Madrakian, & Karimi, 2007). While CC sample was submerged in a washing container and eluted with conductivity water at 60°C for two days, nitrogen gas was applied in the washing container to prevent adsorption of CO2 present in the water. Then, the CC was placed into 4.0 mol L−1 H2SO4 and HCl solutions to generate surface functional groups. Anions are able to electrostatically attach on the CC surface due to the protonation of -OH groups via acid treatment. Nitrite and nitrate are adsorbed from water by interaction between positive charges on the CC surface and negative charges of the anions. Similarly, Mo(VI) and W(VI) were removed from water using acid-treated high surface area CC (Afkhami, Madrakian, & Amini, 2009). In another study, acid treated carbon cloth was hydrothermally modified with porous -Fe2O3 nanoparticles by Tai group (Mahesh, Shown, Chen, Chen, & Tai, 2018). A mixture of HNO3 and H2SO4 (3:1) was used to pretreat the CC surface at 80°C for 2 h. Then, synthesized α-Fe2O3 was attached to the CC surface due to the hydroxyl and carboxyl groups. Finally, they used highly conductive modified CC to detect dopamine. Xu et al. developed an electrochemical sensor based on Au nanoparticles/polyaniline/CC for detection of glucose (Xu et al., 2017). CC was incubated with the use of a 1.0 mM HClO4 solution and PANI clusters were formed at 40 Acm−2 for 3 h. After that, PANI/CC was used as a supporting electrode to deposit AuNPs to the surface. The modified flexible sensor was successfully applied for detection of glucose in human serum. Thus, acid treatment of CC is useful to modify electrode surfaces with nanomaterials and developing sensors for detection of biomarkers and heavy metals.
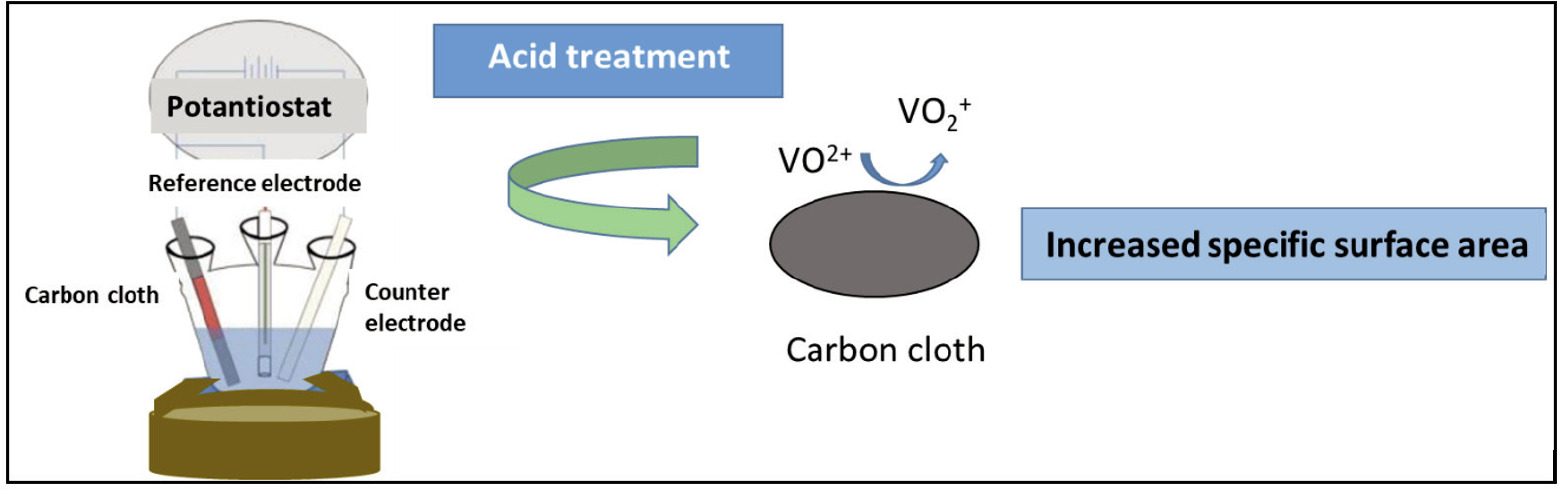
Here, new, disposable, low-cost and simple pretreated CCEs with different functional groups using single step electrochemical activation are presented for the first time. The functionalized electrodes were characterized via cyclic voltammetry (CV) (Scheme 1).
Schematic representation of the acid functionalized carbon cloth.
Materials and methods
Without further purification, all chemicals and reagents were used as obtained. CC was purchased from the Freudenberg Group (Germany). The electrochemical measurements were performed using a potentiostat (Gamry). A traditional 3-electrode system using disposable carbon cloth, a saturated Ag/AgCl electrode and Pt electrode were utilized for electrochemical studies. CV measurements were carried out using 5 mM Fe(CN)63−/Fe(CN)64− in 0.1 M KCl solution. Solutions were prepared with deionized (DI) water (18.2 MΩ).
Electrochemical activation of CC
The electrochemical activation process of commercial CC was conducted using three-electrode configuration in different acidic solutions, including 0.1M H2SO4, 0.1M HCl and 0.1M HNO3, to obtain different functional groups on the surface of the gas diffusion layer (Fig.1). At first, the carbon cloths were placed into specially designed sample holders. 5 cm2 surface area of the CCE had contact with the solution when it was immersed into the coating bath, and an electrochemical activation process occurred on this surface. The activation process was carried out by applying a constant 100 mA current (galvanostatic) for different durations. After the activation process, the gas diffusion layer was taken out from the sample holder, followed by washing with distilled water and drying under a halogen lamp. The geometric surface area of acid treated CC electrodes is around 0.196 cm2.
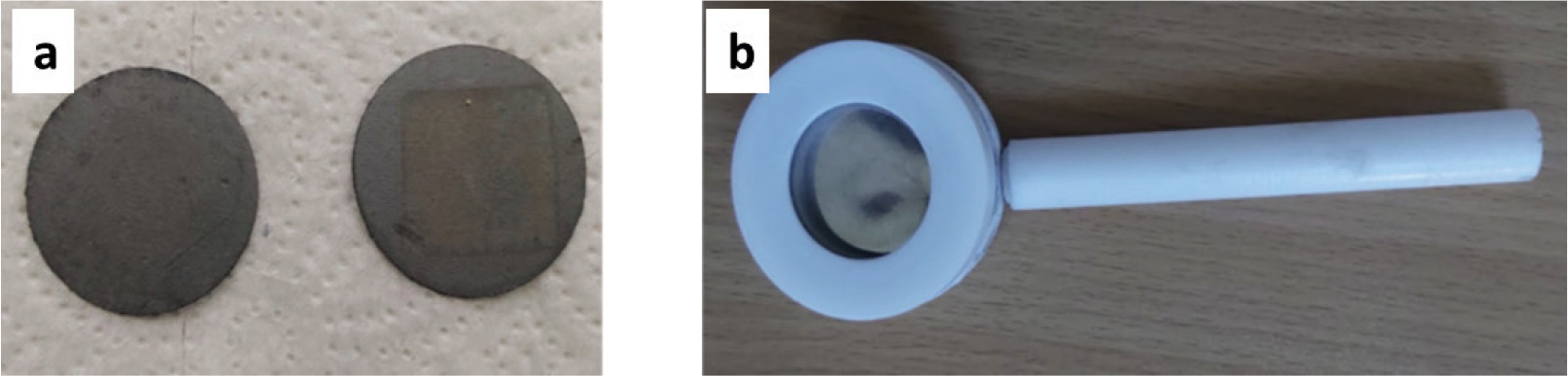
Figure 1. (a) Acid treated carbon cloth, (b) sample holder.
Results and discussion
Determination of electroactive surface area of the CCE
The commercial carbon cloth was electrochemically activated in acid solutions (H2SO4, HNO3, HCl) at a constant current for 10 s, 20 s and 30 s, respectively. The acid treatment of the CCEs with H2SO4 and HNO3 provides the hydroxyl and carboxylic groups on the surface of the electrodes, resulting in improvement of the CC hydrophilicity (Zhang, Yu, Shen, & Hu, 2020).
The electrochemical behavior of the bare and acid treated CCEs was investigated using cyclic voltammetry in 5 mM Fe(CN)63−/Fe(CN)64− in 0.1 M KCl solution. The voltammograms of the electrodes were recorded at various scan rates between 10 mV s−1 and 300 mV s−1 and are shown in Fig. 2-9a. The anodic peak current densities (Ipa) of bare CCE, H2SO4 treated CCE via electrodeposition for 10 s, 20 s and 30 s, HCl treated for 10 s, 20 s, HNO3 treated for 10 s, 30 s at a potential window between -0.1 and 0.6 V with a scan rate of 50 mV/s are 0.32, 1.28, 1.0, 1.05, 0.66, 0.65, 1.28, and 1.22 mA cm−2, respectively. The electroactive surface area of the bare and acid treated CCEs were calculated by using Ipa values in the Randles-Sevcik Equation (Equation 1) (Carvalho, Gouveia-Caridade, & Brett, 2010).
Figure 2. (a) CV of bare CCE versus scan rate from 10 to 300 mV s−1, (b) The square root of the scan rate vs. Ipa at different scan rates for bare CCE.
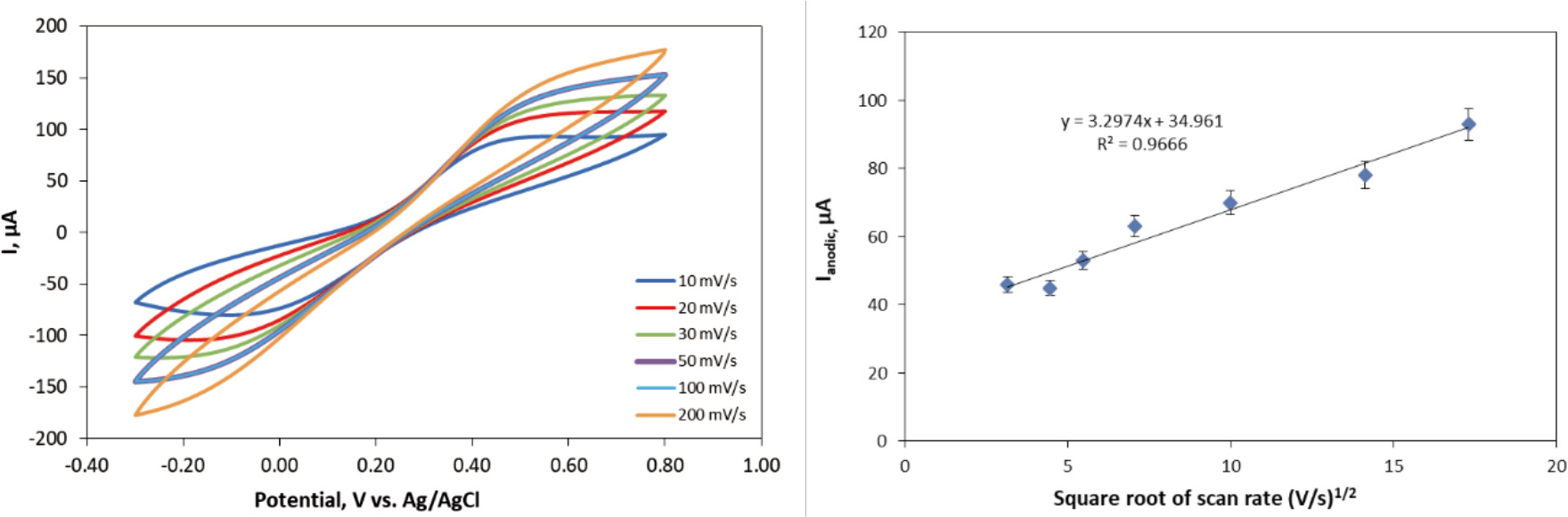
The electroactive surface area of the bare CCE was ~4 times lower than that of the acid treated CCE. Higher background current responses were obtained with the acid treated CC compared to the bare CC electrodes due to the nature of acid treated carbon materials (Luo, Shi, Li, Gu, & Zhuang, 2001). In addition, the optimum duration for acid treatment was found to be 20 s for H2SO4 whereas 10 s for HCl and HNO3.
Previously, it was reported that different functional groups were generated, affecting adsorption or catalysis and the formation of electric double layers because of the interaction between carbons with different complexes (Nian & Teng, 2002; Noh & Schwarz, 1990). The capacitance increased due to the adsorption of ions (McCreery, Cline, McDermott, & McDermott, 1994). Oxygen functional groups are generated on carbon surfaces in the presence of oxidizing acid solutions, such as nitric acid or sulfuric acid via treatment. Therefore, oxygen functional groups offer redox activity to improve the pseudo-capacitance (Koresh & Soffer, 1977).
Electrochemical characterization of CCE
A 5 mM [Fe(CN)6]3−/4− electrolyte solution including 0.1 M KCl was utilized in order to electrochemically characterize the CCE. The effect of the scan rate on the peak currents was evaluated in Fig. 2-6, which shows CV curves of bare CCE and acid treated CCE. As can be depicted in Fig. 2-6, current responses increased with increasing scan rate from 10 mV s−1 to 300 mV s−1 , demonstrating diffusion-controlled reaction process (Adhikari et al., 2017). While the reduction peaks were observed at negative potentials, the oxidation peaks were at positive potentials with the increase in the scan rates. The ion diffusion for electronic neutralization during a rapid faradic reaction might lead to an increase in internal diffusion resistance (Zhao et al., 2019).
Figure 3. (a) CV of H2SO4 treated (10 s) CCE versus scan rate from 10 to 300 mV s−1, (b) The square root of the scan rate vs. Ipa for 0.1 M H2SO4 treated (10 s) CCE. (c) CV of H2SO4 treated (20 s) CCE versus scan rate from 10 to 300 mV s−1, (d) The square root of the scan rate vs. Ipa for 0.1 M H2SO4 treated (20 s) CCE. (e) CV of H2SO4 treated (30 s) CCE versus scan rate from 10 to 300 mV s−1, (f) The square root of the scan rate vs. Ipa for 0.1 M H2SO4 treated (30 s) CCE.
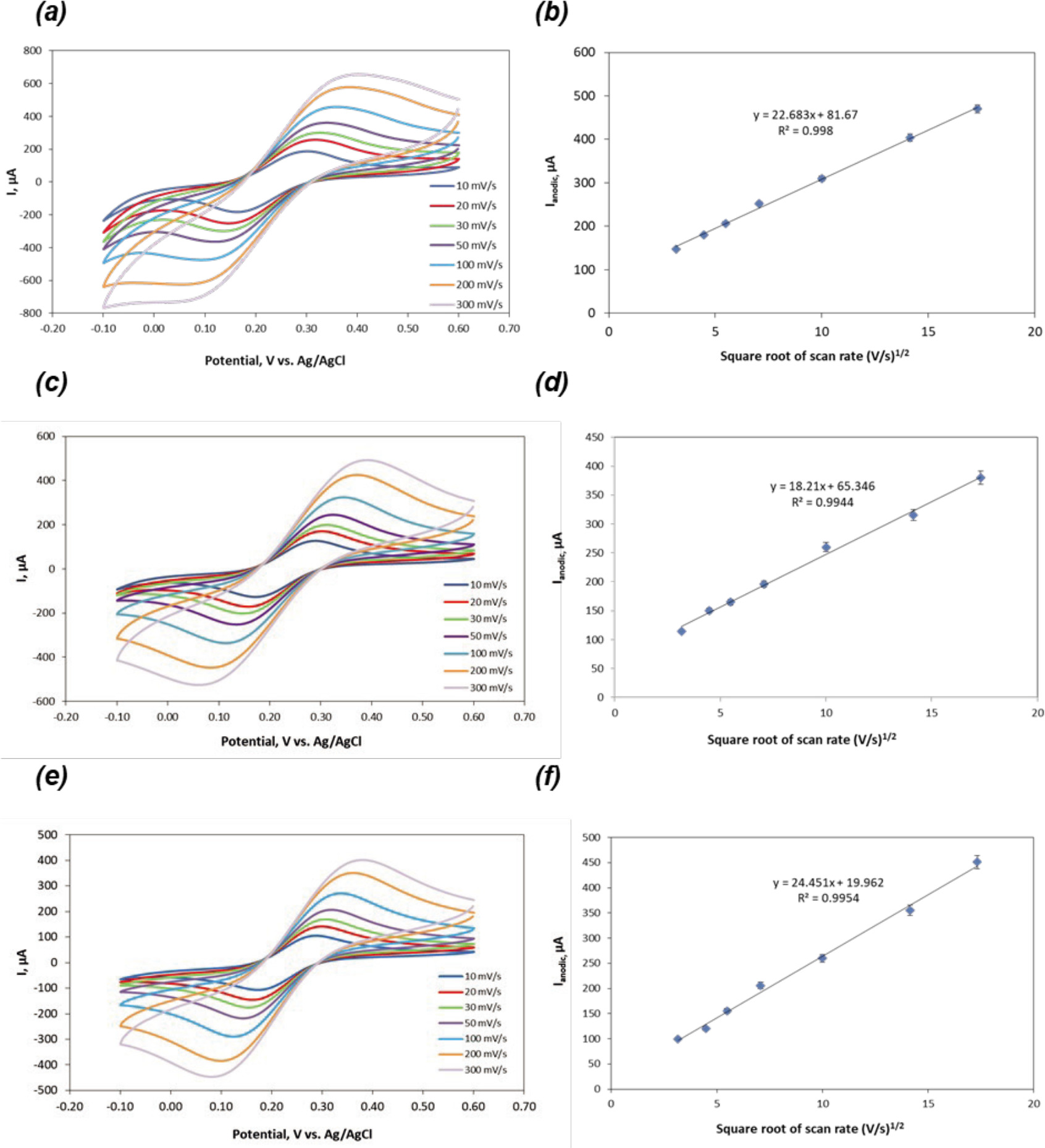
Figure 4. (a) CV of HCl treated (10 s) CCE versus scan rate from 10 to 300 mV s−1, (b) The square root of the scan rate vs. Ipa for 0.1 M HCl treated (10 s) CCE. (c) CV of HCl treated (20 s) CCE versus scan rate from 10 to 300 mV s−1, (d) The square root of the scan rate vs. Ipa for 0.1 M HCl treated (20 s) CCE.
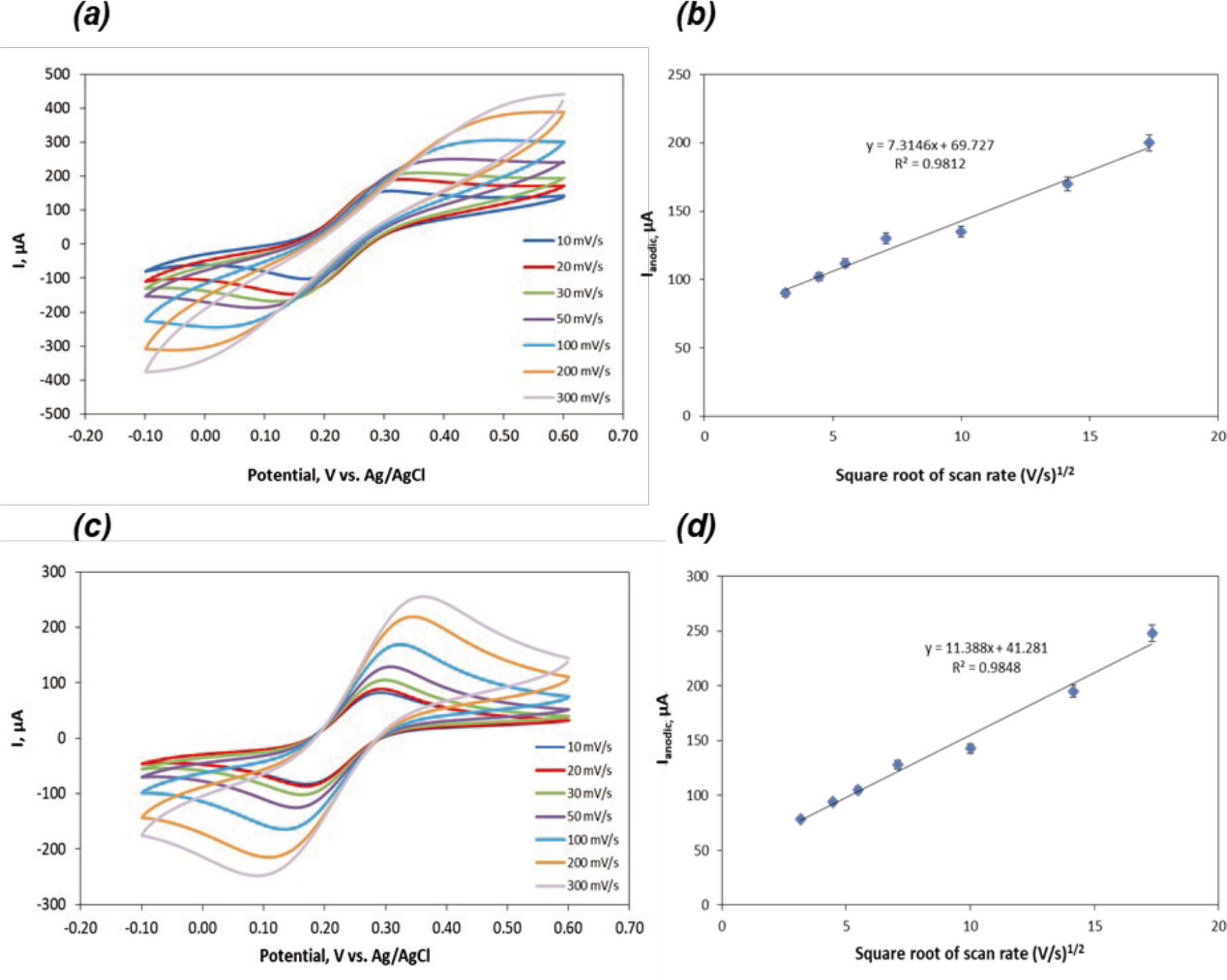
Figure 5. (a) CV of HNO3 treated (10 s) CCE versus scan rate from 10 to 300 mV s−1, (b) The square root of the scan rate vs. Ipa for 0.1 M HNO3 treated (10 s) CCE. (c) CV of HNO3 treated (30 s) CCE versus scan rate from 10 to 300 mV s−1, (d) The square root of the scan rate vs. Ipa for 0.1 M HNO3 treated (30 s) CCE.
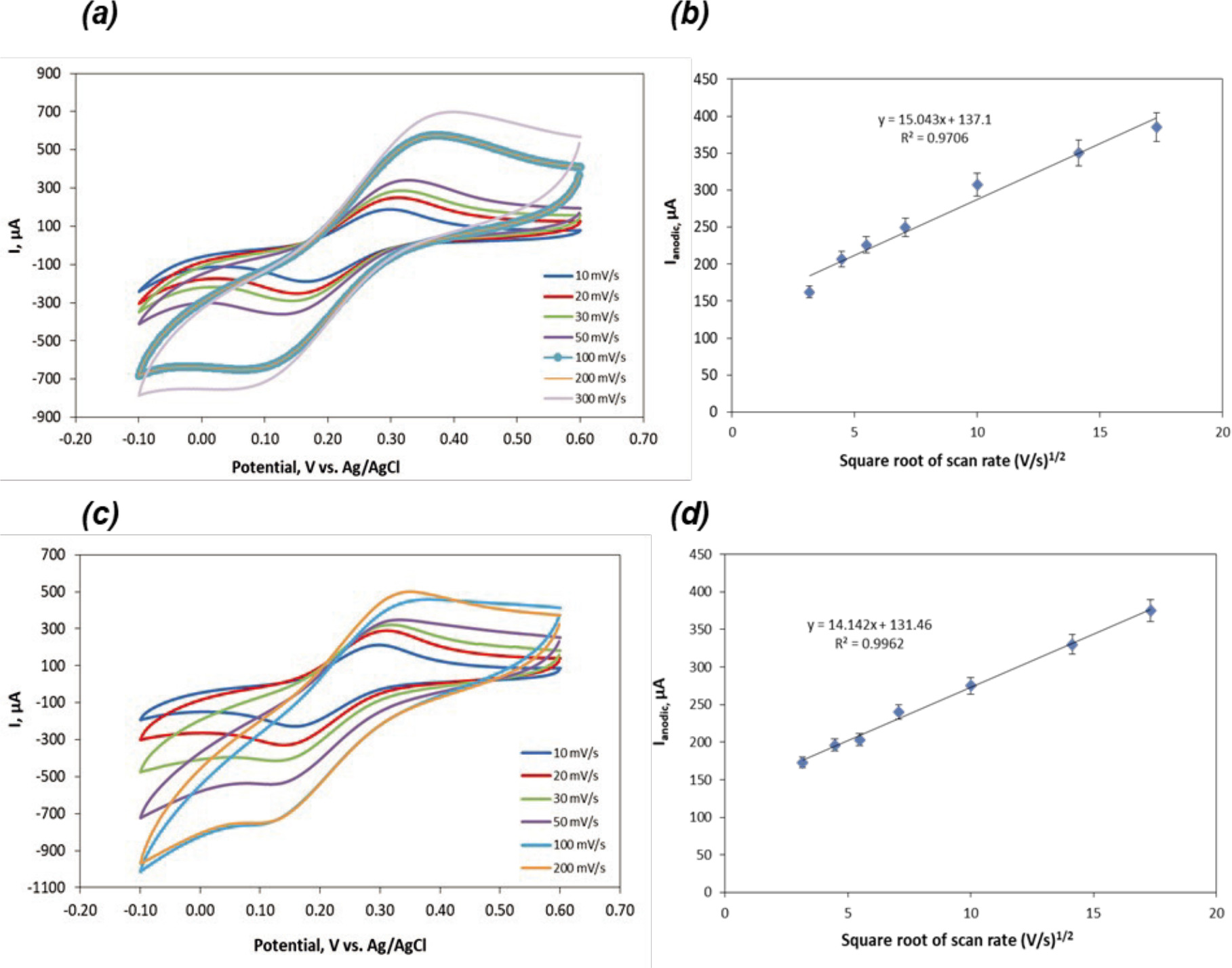
Figure 6. CV of bare CCE and acid treated CCEs at the scan rate of 50 mVs−1.
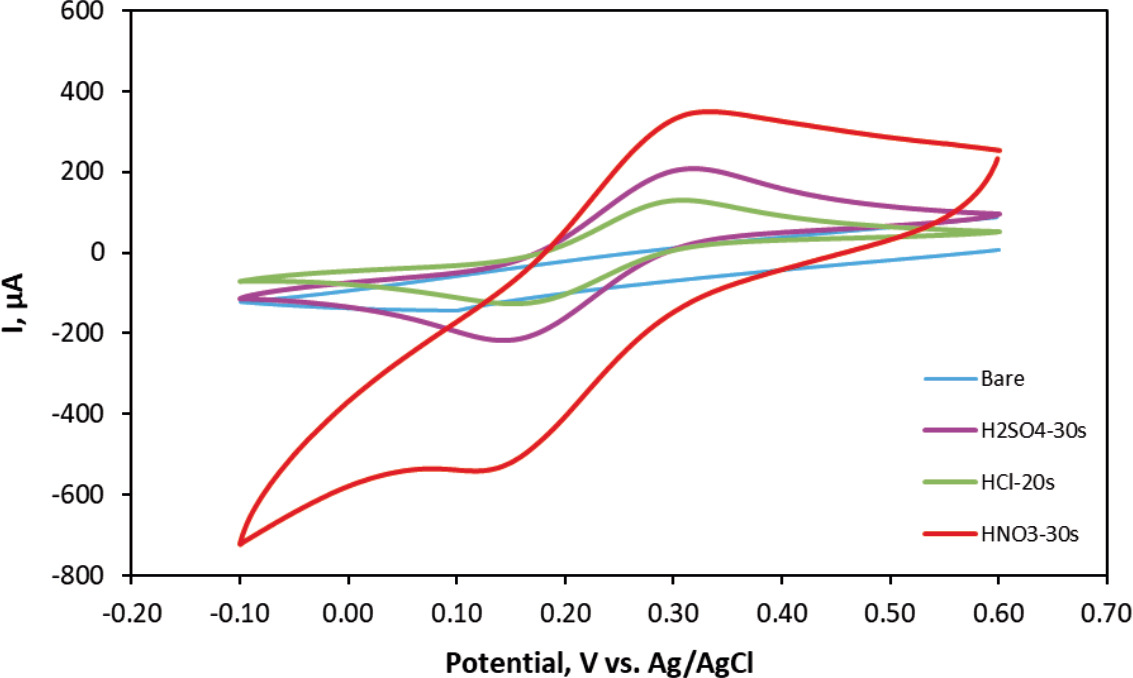
Acid treated CCE exhibited a higher redox peak and improved electrochemical responses compared to bare CCE. Since CC has higher active surface area and better conductivity than bare CCE, the electron transfer process may be improved (Shi et al., 2019).
It was observed that the CV area of HNO3 treated (30 s) CCE was much larger than those of the H2SO4 (30 s) and HCl (20 s) treated CCE and bare CCE (Fig.10). Moreover, HNO3 (30 s), H2SO4 (30 s) and HCl (20 s) treated CCs demonstrated similar reaction reversibility and reaction kinetics behavior.
Conclusions
A simple and inexpensive electrodeposition method to functionalize carbon cloth substrate is presented in this study. Oxidation through nitric acid and sulfuric acid treatment increased the electrochemical capacitance of activated carbon cloth electrodes. Cyclic voltammetry demonstrated that the presence of the oxygen desorbing complexes enhanced the double-layer formation, as well as the capacitance. Thus, the capacitance improvement can be attributed to the increased presence of the CO-desorbing complexes. Consequently, the faradaic current increased with the total number of oxygen atoms on the electrode surface, enhancing the redox process. Also, the modified electrodes showed fast electron transfer and good reversibility (Wu et al., 2023). Acid modified disposable CC electrode holds the potential for the development of flexible biosensors to detect analytes in various application fields.