Introduction
Cancer remains one of the most formidable health challenges in modern medicine, with a cure still eluding scientists despite extensive research efforts. Studies have shown that conventional treatments cause serious side effects and toxicity, and in some cases, resistance to chemotherapy and radiotherapy can develop (Dolatkhah Laein et al., 2023). Therefore, it is important to develop new treatment strategies. In recent years, natural compounds have emerged as promising candidates for cancer treatment, leading to a surge in research focused on their therapeutic potential.
Flavonoids have been shown to have antioxidant and radical-scavenging properties on many diseases including cancer (Lin et al., 2012). Rutin, one of the flavonoids, is a glycoside containing rutinose attached to the OH-3 of quercetin (Agrawal et al., 2021). It has been shown that rutin has antioxidant, anti-inflammatory, anti-aging, and anticancer properties (Choi et al., 2021; Choi et al., 2016) and that it can be used as a protective drug since no toxicity was observed during long-term use (Youssef et al., 2022).
Krill oil (KO), extracted from Euphausia superba, is seen as an alternative to fish oil because it contains high levels of long-chain omega-3 polyunsaturated fatty acids (LC n-3 PUFA) (Tou et al., 2007; Winther et al., 2011). Various studies suggest that KO may have therapeutic effects in the treatment of many diseases, including various types of cancer. One study suggests that KO may suppress tumor growth in human colorectal cancer (CRC) (Jayathilake et al., 2022). In another study, it was reported that KO can regulate both tumor growth and tumor-associated angiogenesis via a novel mechanism (Hoon et al., 2022). It has also been found that the anti-proliferative property of KO is comparable with that of a chemotherapeutic drug, oxaliplatin (Jayathilake et al., 2019).
Despite the demonstrated efficacy of rutin and krill oil in cancer therapy both in vitro and in vivo, their clinical applications are limited due to several challenges such as poor water solubility, low absorption, and rapid metabolism (Manach et al., 2005; Nguyen et al., 2013; Sanchez et al., 2021). To overcome these limitations, nanotechnology has introduced innovative solutions, including the development of advanced DDSs. These nanocarrier systems are designed to enhance the solubility, stability, and targeted delivery of plant-based therapeutics, thereby improving their efficacy and applicability in cancer therapy. To overcome these challenges and achieve targeted cancer therapy, various drug delivery systems (DDSs) have been developed (Dobrzynska et al., 2020; Zhao et al., 2020; Naeem et al., 2023).
Carbon-based nanomaterials (CBNs) are among the carrier materials that attract attention. CBNs have high chemical resistance, efficient mechanical properties and biocompatibility properties (Demirhan et al., 2021; Zhang et al., 2016). Carbon nanofibers (CNFs) attract attention due to their different properties and applications (Magrez et al., 2006). The addition of CNFs to calcium alginate composite hydrogels can lead to an increase in mechanical properties and water diffusion (Llorens-Gamez and Serrona-Aroca, 2018). Alginates are of interest in drug delivery applications due to their biocompatibility and low toxicity properties (Putri et al., 2021).
In our study, we aim to develop rutin modified carbon nanofiber-based aerogels loaded with KO as a novel therapeutic approach. These aerogels are not only evaluated for their antiproliferative effects on various cancer cell lines, but also for their antimicrobial activities, providing a dual-functional platform for cancer treatment.
Material and methods
Reagents
The CNF was provided by Sigma Aldrich (>98% carbon basis), rutin (≥95%) was supplied by Aromel Chemistry (Konya, Türkiye), and KO was obtained from TAB Pharma (İstanbul, Türkiye). Dulbecco’s Modified Eagle Medium/F12 (DMEM/F12) was purchased from PAN Biotech (Aidenbach, Germany). DMEM and fetal bovine serum (FBS) were obtained from Gibco (Rockville, MD, USA), MTT Cell Proliferation Assay Kit was provided by Thermo Fisher Scientific (Waltham, MA, USA). All other chemicals were obtained from Sigma-Aldrich (United Kingdom). All chemicals were of analytical grade purity.
Activation of carbon nanofibers (CNFs)
CNFs were washed and purified from impurities by stirring with deionized water (DI) for 24 hours on a magnetic stirrer and then dried. Then, they were subjected to an acid treatment to convert them into active CNFs (ACNFs). Briefly, 3 g of CNF was soaked in 50 mL of 85% H3PO4 for 24 hours at room temperature and left at 250°C to active CNF. The CNF was centrifuged with 50 mL of DI water at 5000 rpm for 30 minutes. This process was repeated until the pH became neutral. Once the pH was stabilized, it was dried at 85°C to obtain active carbon nanofibers (ACNFs) (Bingol Ozakpinar et al., 2023).
Modification of ACNFs with rutin (ACNFr)
300 mg of rutin was dissolved in 2 mL of dimethyl sulfoxide (DMSO) until completely dissolved. 300 mg ACNF dispersion were dispersed DI water with ultrasonic water bath. The rutin solution was added to the ACNF dispersion in a total volume of 200 mL DI water and incubate at 37 °C in a shaking water bath for 24 hours. This mixture was centrifuged, washed with DI water, and then dried at 37 °C.
Preparation of aerogels
To prepare aerogels, 4 mg of ACNFr was dispersed in 8 mL of DI water and added 1 mL of KO (dissolved in 1 mL of DMSO) to prepare a 0.4 mg/mL ACNFr-KO mixture. The mixture was added dropwise into 10 mL of sodium alginate solution (2 g/100 mL DI water). After stirring for 10 minutes with a magnetic stirrer, it was then added dropwise into 3 g of CaCl2/100 mL DI water solution and allowed to stand at room temperature for 24 hours. For the ACNFr and ACNF dispersions, the identical procedure was used once more. The resultant aerogels were cleaned with distilled water, allowed to stand at room temperature for a full day, and then dried at 37 °C.
Characterization of aerogels
The scanning electron microscope (SEM) was utilized to determine the surface structure and morphological features of the composite aerogels (Zeiss EVO MA 10). Prior to imaging, nanoparticles underwent a gold-palladium coating process using Quorum SC7620 Mini Sputter Coater for 180 seconds. An acceleration voltage of 10 kV was applied. The IRSpirit spectrometer (Shimadzu Corp, Kyoto, Japan) was used to record the FTIR (Fourier transform infrared) spectra of the samples. Measurements were taken in the range of 4000−450 cm−1 with an average resolution of 4 cm−1.
Cell culture studies
In this study, human colorectal cancer cell line (HT29- ATCC, HTB-38), human breast cancer cell line (MCF-7-ATCC, HTB 22), human cervical cancer cell line (Hela-ATCC, CCL2), and human prostate cancer cell line (PC3-ATCC, CRL1435) were used. The cells were cultured in DMEM medium supplemented with 10% FBS and 1% penicillin-streptomycin at 37°C and 5% CO2.
Cell viability assessment
The effects of ACNF, rutin, KO, and aerogels on HT29, MCF-7, Hela, and PC3 cells on cell viability were determined using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) method (Beekman et al., 1996). Briefly, the cells were seeded in 96-well plates and cultured for 24 hours. The next day, ACNF (10, 50, 100 µg/mL), rutin (100, 200, 300 µg/mL), KO (10, 40, 80 µg/mL), rutin-modified ACNF (10, 50, 100 µg/mL) and aerogel (10, 50, 100 µg/mL) were applied to the cells. After incubation, After adding MTT solution to the wells, the cells were cultured for four hours in a CO2 incubator. Using 630 nm as the reference wavelength, the optical density (OD) was measured at 570 nm in a multi-well plate reader (Biotech Instruments, Winooksi, VT, USA). All experiments were run twice, and each treatment was performed in triplicate. The following formula was used to determine cell viability:
[(Average OD of treated cells) / (Average OD of control cells)] × 100
Evaluation of antimicrobial activity of aerogels
The antimicrobial properties of the rutin-modified composite aerogels and KO-loaded aerogels were investigated using the agar diffusion test after 30 minutes of UV application. Later, also the minimal bactericial concentrantion (MBC)s and minimal fungisidal concentrantion (MFC)s of the aerogels were assessed.
Agar well diffusion test: As bacteria; Enterococcus faecalis ATCC 29212, Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922, Staphylococcus epidermidis ATCC 11228 and as yeast; Candida albicans ATCC 90028 strains were used. Bacteria were inoculated on Mueller Hinton Agar (MHA) 37 °C for 24 hours and C. albicans on Sabouraud Dextrose Agar (SDA), incubated at 35 °C for 48 hours. Colonies of microorganisms were cultivated in 0.85% NaCl physiological saline solution (PSS) to make up microorganism suspensions. Using the McFarland 0.5 standard turbidity as a guide, bacterial suspensions were adjusted to 108 CFU/ mL and C. albicans suspensions to 106 CFU/mL. Using sterile swabs and aseptic circumstances, the microorganism suspensions were applied to the surface of the MHA for bacteria and the SDA for C. albicans. Next, 5 mm diameter wells were formed on the medium using a sterile punch. Next, 50 µL of the aerogels were added to the wells. Additionally, amphotericin B was utilized as a positive control for the yeast, meropenem was used for the bacteria, and PSS and solvent (DMSO) were used as the negative controls. Inoculated petri dishes were incubated for 18–24 hours at 37 °C for bacteria and 24–48 hours at 35 °C for yeast. The inhibition zones were measured in millimeters at the end of the incubation period. Three repetitions of the trials were conducted, and the average range of those replicates were determined (Peretz et al., 1990; Ritz et al., 1993; Balouiri et al., 2015).
Detection of minimal inhibitor concentration (MIC) for bacteria: The Clinical and Laboratory Standards Institute (CLSI) guidelines were followed while determining the minimum inhibitory concentration (MIC) of bacteria. The medium utilized was Cation Adjusted Mueller Hinton Broth (CAMHB). A bacteria suspension was prepared from the colonies in the 24-hour bacterial culture according to McFarland 0.5 turbidity, and the final inoculum concentration was diluted to 5x105 CFU/ mL. The sterile U-based microdilution plates were placed 100 µL of the CAMHB. A quantity of 100 µL aerogel samples were placed in the first wells and serial dilutions were made respectively. Then 5µL of bacterial suspension was added to the wells containing the aerogels and the plates incubated at 37 °C for 24 hours (CLSI 2020).
Detection of MIC for yeasts: The suspensions of yeasts, prepared from 18-24 hour agar cultures with physiological saline according to Mac Farland 1 standard, where the yeast suspensions were diluted to 2×103 CFU/mL and used in the experiment (CLSI 2012, Berkow et al.,2020). Initially, 100 µL of RPMI 1640 medium for yeasts were added to each well of the sterile 96-well microplates. Subsequently, 100 µL of aerogel samples were introduced into the first wells of the plates, followed by 12 serial dilutions. Finally, the prepared Candida albicans suspension was added to all wells, and the microdilution plates were incubated at 35°C for 24 – 48 hours. (CLSI 2012, Berkow et al., 2020). At the end of this period, the lowest concentration of aerogels at which no growth of any microorganisms was observed was determined to be the minimum inhibitory concentration (MIC) value. Likewise, CAMHB, DMSO and RPMI were used as negative control. Meropenem and Amphotericin B were used as positive control (CLSI 2020; CLSI 2012; Berkow et al., 2020).
Detection of minimal bactericidal and fungisidal concentrations: Seeding was performed from each well of the microplate dilution containing the MIC value on Tryptic Soy Agar and Sabouraud Dextrose Agar in order to determine the minimal bactericidal concentration (MBC) and minimal fungicidal concentration (MFC) values of the aerogel samples. Then, the plates were left to incubation at 37 °C for 24 hours for bacteria and 48 hours for the yeast. Finally, after the incubation, the MBC and MFC values were determined according to the lowest values where no growing was seen observed (Misra and Sahoo, 2012).
Results and discussion
Characterization of aerogels
Characterization of the nanofibers and aerogel samples were done by using FTIR analysis and scanning electron microscopy (SEM). FTIR spectra of the ACNF and the aerogel samples were given in Figure 1. There is a stretching band at around 3300 which shows O-H bonds of the alginate matrix of the composite aerogel and does not exist in the spectra of ACNFr. There is a sharp peak around 1600 cm-1 belongs to C=O in the spectra of A(ACNFr). The peaks between 1500 cm-1 and 1000 cm-1 correspond to C-C and C-O single bonds. There are some changes in the positions of the peaks between 1600 and 100 cm-1 due to the modification of the structure with krill oil. There are also new peaks at around 2900 cm-1 due to C-H stretching as a result of the krill oil modification (Bingol Ozakpınar et al., 2023; Calışkan Salihi et al., 2021; Wang et al., 2017; Demirhan et al., 2021).
Figure 1. FTIR spectra of the ACNFr and aerogels.
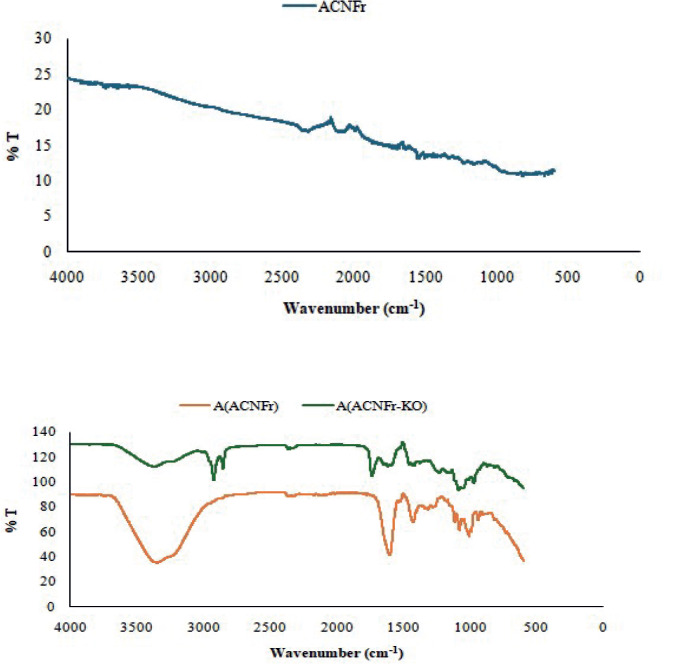
The physical structure and surface morphology of the aerogel samples were investigated by SEM. SEM photographs clearly show the porous and non-uniform physical structure of the aerogels (Figure 2).
Figure 2. Representative SEM images of the aerogel samples. ACNFr (A, B) and ACNFr-KO (C, D).
Antiproliferative effects of ACNF, rutin, KO and aerogel samples on the human cancer cell lines
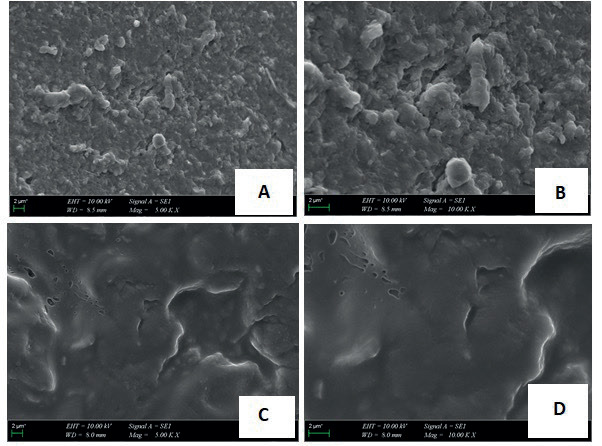
We investigated the antiproliferative effects of ACNF on cancer cell lines in our study. Treatment of HT29, MCF-7, Hela, and PC3 cell lines with increasing concentrations of ACNF inhibited their proliferation in a dose-dependent manner in all cell lines (Figure 3). This inhibitory effect was observed to be particularly pronounced in HeLa cells. Ringel et al. (2014) demonstrated that carbon nanotubes (CNTs) and CNFs sensitized prostate and bladder cancer cells to the platinum-based chemotherapeutic carboplatin (CP) and cisplatin (CDDP). This sensitization resulted in an enhanced inhibition of both short- and long-term proliferation, as well as an increased induction of apoptosis. They also reported that the inhibitory effects of CNTs and CNFs alone on cellular viability were approximately 20%. In our study, this inhibitory effect was observed to be approximately 30% in PC-3 cells. This is likely due to the activation of CNFs. On the other hand, it was determined that the inhibitory effects on cancer cells did not change when ACNF was modified with rutin (ACNFr) or when it was in aerogel form (Aero (ACNFr)) (Figure 4).
Figure 3. The antiproliferative effect of ACNF, rutin, and krill oil on HT-29, MCF-7, PC-3 and HeLa cell lines.
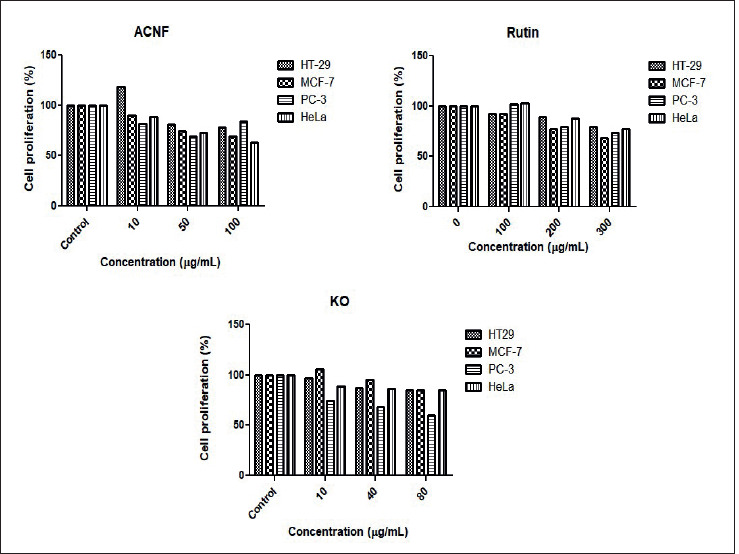
Figure 4. The antiproliferative effect of ACNFr, Aero(ACNFr), and Aero(ACNFr+KO) on HT-29, MCF-7, PC-3, and Hela cell lines.
When rutin was applied at concentrations ranging from 0 to 300 µg/mL, it exhibited approximately a 23% antiproliferative effect in HeLa, HT-29, and PC-3 cells, while reducing cell viability by about 32% in MCF-7 cells (Figure 3). These findings support that rutin has antiproliferative effects in cancer cells. As it is well known, rutin is an anticancer flavonoid, and its efficacy has been demonstrated in numerous in vivo and in vitro studies (Iriti et al., 2017; Satari et al., 2021). Parallel to our study, Irriti et al. (2017) demonstrated the cytotoxic effects of rutin in MCF-7 cells in their research.
Krill oil (KO) is a natural compounds thought to play an effective role in cancer treatment and prevention (Jayathilake et al, 2022). Long-chain n-3 polyunsaturated fatty acids, particularly eicosapentaenoic and docosahexaenoic acids, and the powerful antioxidant astaxanthin are found in krill oil (Liu et al., 2024). In this study, we also evaluated the anti-carcinogenic effects of KO on the cancer cell lines (Figure 3). Each cell line was grown in media containing different concentrations (10, 40, and 80 µg/ mL) of KO. The results revealed that the inhibition of cell viability increased with increasing dose and that KO had an antiproliferative effect. When evaluating the MTT results, we found that rutin applied alone exhibited a higher antiproliferative effect on HT-29, HeLa, and MCF-7 cells compared to KO applied alone. On the otherhand, KO’s antiproliferative effect on PC-3 cells was significantly higher than that of rutin (40%). Interstingly, the rutin-modified aerogel loaded with KO composite, Aero (ACNFr-KO) exhibited a lower antiproliferative inhibition on cancer cells compared to the effect observed with KO alone.
We found that the drug carrier Aero (ACNFr-KO) composite we produced exhibited higher antiproliferative activity on cancer cells compared to Aero (ACNFr) composite across all cell lines. This inhibitory effect was particularly pronounced at the highest concentration of Aero (ACNFr-KO) composite, especially in MCF-7 (64%) and HeLa (68%) cells. The different effects of aerogels on different cell lines may be due to the different biological properties of the cells and the way aerogels interact with the cells.
Determination of antimicrobial activities of aerogels
As can be seen in Table 1, Aero (ACNFr-KO) showed antibacterial activity against E. faecalis ATCC 29212, which is one of the Gram-positive microorganisms, and formed an inhibition zone of 11.21 mm. However, it was found to be ineffective against other microorganisms. Enterococcus faecalis (E. faecalis), which is found in the normal flora of the oral cavity, is a Gram-positive microorganism that plays a primary role in the pathogenesis of recurrent infections (Tariq et al, 2023). This bacterium, which contains virulence factors against host cells, causes approximately 80 to 90% of hospital-acquired infections attributed to enterococci. As an opportunistic pathogen, E. faecalis has the opportunity to cause serious diseases, sometimes with fatal consequences, especially when the host’s immune system is weakened. It is also responsible for the development of chronic infections such as surgical wound infections, bacteremia, endodontic infections, infective endocarditis, abdominal infections, and urinary tract infections (Ardila et al., 2023).
Table 1. Inhibition zones of aerogel composite systems determined by agar well diffusion method (mm).
| Enterococcus faecalis ATCC 29212 | Staphylococcus aureus ATCC 25923 | Staphylococcus epidermidis ATCC 11228 | Pseudomonas aeruginosa ATCC 27853 | Escherichia coli ATCC 25922 | Candida albicans ATCC 90028 | |
|---|---|---|---|---|---|---|
| ACNF | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| ACNFr | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| A(ACNFr) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| A(ACNFr+KO) | 11.21 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| QRILL OIL | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| RUTIN | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Meropenem (10μg/mL) | 18.32 | 32.80 | 42.73 | 29.10 | 30.12 | 0.00 |
| Amphotericin B (100μg/mL) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 22.73 |
As seen in Table 2, the MIC of Aero (ACNFr-KO) was determined to be 50 mg/mL against E. faecalis ATCC 29212. The MBC of Aero (ACNFr-KO) was also found to be 100 mg/mL against E. faecalis ATCC 29212. However, this aerogel did not have any effect on the other microorganisms in our study. We have not found any study related with the antimicrobial effect of Aero (ACNFr+KO). Accordingly, our findings mihgt be useful in the area related with aerogel composite systems, which were mentioned above.
Table 2. Detection of antimicrobial activity of aerogels by microdilution (μg/mL ).
| Enterococcus faecalis ATCC 29212 | Staphylococcus aureus ATCC 25923 | Staphylococcus epidermidis ATCC 11228 | Pseudomonas aeruginosa ATCC 27853 | Escherichia coli ATCC 25922 | Candida albicans ATCC 90028 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| ACNF | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| ACNFr | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| A(ACNFr) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| A(ACNFr+KO) | 50 | 100 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| KRILL OIL | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| RUTIN | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Meropenem (10μg/mL) | 8 | 16 | 2 | 4 | 0.25 | 0.50 | 0.5 | 2 | 0.06 | 0.12 | 0.00 | 0.00 |
| Amphotericin B (100μg/mL) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1 | 4 |
Conclusion
Drug delivery systems (DDS) play an important role in cancer treatment by providing effective delivery of drugs to the target site and reducing side effects. This study investigates antiproliferative and antimicrobial effects of rutin and KO using alginate aerogel nanoparticles. Our study suggests that by using natural products such as rutin and KO, it is possible to develop more effective treatment strategies by delivering drugs to cancer cells. Further studies are needed on different types of cancer. Additionally, the antimicrobial activity of ACNFr-KO aerogels suggests their potential for drug development. Targeted delivery of rutin using ACNFr aerogels has the potential to offer a new treatment strategy for cancer and infection therapy.