Introduction
The formation of biofilms by group-forming bacteria is a significant phenomenon with implications across various fields. Biofilms, which are intricate communities of microorganisms adhering to surfaces, contribute to processes such as nutrient cycling, bioremediation, and pathogenesis (Bridier et al., 2011; Davey & O’toole, 2000; Flemming & Wingender, 2010; Hall-Stoodley et al., 2004; Stewart & Franklin, 2008). Microbial fuel cells (MFCs) are biotechnological devices capable of generating electricity, while treating wastewater. A MFC typically consists of an anode, a cathode, a separator (proton exchange membrane or PEM), and an external circuit for electron flow (Asensio et al., 2016; Kim et al., 2007). In the anode, microorganisms oxidize organic or inorganic substrates, producing electrons. These electrons are transferred to the cathode through an external circuit, where they react with protons and oxygen to generate water, producing electrical power (Fan et al., 2024; Quan et al., 2012; Sonmez et al., 2024; Wang et al., 2011; Zhuo et al., 2011).
The relationship between group-forming bacteria and MFCs is vital, as biofilms formed by these bacteria significantly influence MFC performance and efficiency. Biofilm-forming bacteria, including those from genera such as Bacillus, Pseudomonas, and Geobacter, dominate MFC biofilms, facilitating electron transfer and enhancing substrate utilization (Rabaey et al., 2005; Logan et al., 2006; Huang & Logan, 2008).
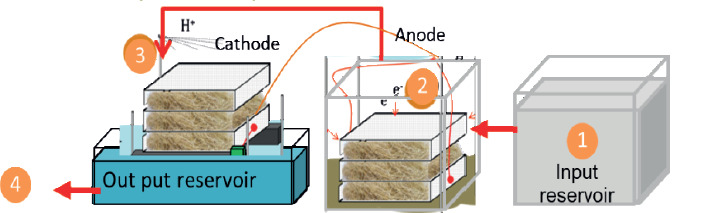
The BioCircuit: biocatalyst microbial fuel cell, developed in previous studies (Sukkasem et al., 2008, 2011; Sukkasem & Laehlah, 2013, 2015), aims to treat nutrients like nitrate and sulfate in wastewater. The configuration, adapted from the UBFC single chamber to double chambers, maintains the concept of membrane-less operation and avoids platinum catalysts. Electrons and protons transfer from the anode to the cathode via a circuit and a pipe, facilitating reactions crucial for wastewater treatment (Figure 1).
Figure 1. Schematic of the BioCircuit system 1) input reservoir 2) the circuit for the electron transfers from anode to cathode 3) the pipe for protons transferring form anode to cathode 4) output reservoir.
Anode reaction (in the water) : C6H12O6 + 6H2O →6CO2 + 24H++ 24e- [1]
Cathode reaction (on the water) : 6O2 + 24H++ 24e− →12H2O [2]
Total reaction: C6H12O6 + 6O2 →6CO2 + 12H2O [3]
Natural rubber is a pivotal material in our economy and daily lives due to its exceptional properties, such as strength, surpassing those of synthetic rubber. However, the production process of natural rubber from raw latex results in substantial wastewater discharge. This rubber processing wastewater is typically contaminated with sulfur compounds stemming from sulfuric acid coagulation, preservatives, and inherent latex constituents. Under anaerobic conditions, sulfate reduction occurs, leading to the release of toxic hydrogen sulfide gas (H2S). The emission of H2S not only poses significant health and environmental hazards, but also incurs economic repercussions due to its corrosive effects on metallic structures. The discharge of such wastewater into groundwater reservoirs has severe consequences, particularly in southern Thailand (Sukkasem & Laehlah, 2015).
The biocatalyst microbial fuel cell demonstrates remarkable efficacy in treating inorganic substances like sulfate and sulfide, which serve as precursors to malodorous and hazardous hydrogen sulfide gas. It facilitates the conversion of soluble sulfate and sulfide into solid forms, such as elemental sulfur or pyrite (Eq. 4-Eq. 8), which precipitate within the system and can be easily separated from the wastewater stream.
Anode reaction;
SO42− + 10H+ → H2S + 4H2O + 10e− [4]
H2S + 2e− → S0 + 2H+ [5]
SO42− + 8H+ → S0 + 4H2O + 8e− [6]
Fe2+ + 2SO42- + 2H+ → FeS2 + 7/2 O2 + H2O [7]
The reaction for pyrite formation is shown in eq (7) which occurs both in anode and cathode when the excess sulfate and iron ions present:
Cathode reaction:
Fe2+ + 2SO42− + 16H+ → FeS2 + 8H2O [8]
The formation of pyrite (iron sulfide, FeS2) involves a chemical reaction that can occur both at the anode and cathode under specific conditions when there is an excess of sulfate and iron ions present. This reaction is crucial to understanding various contexts, including geochemistry, environmental science, and electrochemistry. In electrochemistry, the formation of pyrite can occur in electrochemical cells under certain conditions. For example, in microbial fuel cells (MFCs) or other bioelectrochemical systems, sulfate-reducing bacteria can catalyze the reduction of sulfate to sulfide at the cathode, where iron ions may be present. This can lead to the precipitation of pyrite on the cathode surface, affecting the performance and longevity of the electrochemical system. Overall, discussing the reaction for pyrite formation provides insight into a complex interplay of chemical processes that occur in various natural and engineered systems. Understanding these reactions is essential for addressing environmental challenges, optimizing industrial processes, and advancing our knowledge of Earth’s geochemical cycles.
Consequently, the rubber process wastewater treatment by the BioCircuit was investigated in this study to determine the performance, efficiency, and feasibility for practical application.
Materials and methods
Sample collection and bacterial cultivation
Rubber wastewater sludges (10 g) were obtained from two sampling sites within a rubber wastewater treatment plant located in Phatthalung province, Thailand. The sludge samples were inoculated into 100 mL of sterile nutrient broth (1 g/L beef extract, 2 g/L yeast extract, 5 g/L peptone, and 5 g/L sodium chloride), supplemented with 50 mg/L nystatin to eliminate fungal contamination. The nutrient broth was then incubated for 24 hours at room temperature with agitation at 150 rpm.
Bacterial isolation
The incubated broth from the previous step was streaked onto nutrient agar plates (1 g/L beef extract, 2 g/L yeast extract, 5 g/L peptone, 5 g/L sodium chloride, and 15 g/L agar) and incubated for 24 hours at room temperature. The cultures were streaked repeatedly until pure cultures were obtained.
Microscopic screening and motility test
Pure cultures were examined under light microscope to identify group-forming bacterial shapes, and those exhibiting such characteristics were selected. The motility of these cultures was determined using the swarming motility test, which assesses rapid multicellular bacterial surface movement.
Identification
Genomic DNA was extracted from selected bacterial strains using the Bio-Rad DNA Extraction Kit (Biorad, United States). The 16S rRNA gene was amplified using the universal primer pair Bact-0341 (CCTACGGGNGGCWGCAG) and Bact-0785 (GACTACHVGGGTATCTAATC). The PCR products were sequenced, and the obtained sequences were compared to sequences in the GenBank database for identification.
Electricity generation property test
Each group-forming bacterial culture was grown in potato dextrose broth for 3 days. The half-cell potential and electricity generation properties were tested using an Ag/AgCl reference electrode and microbial fuel cell (MFC), respectively. In the MFC test, each bacterial culture was introduced into the anode compartment, and the voltage of each MFC was recorded using a data logger program at intervals of 1000 milliseconds.
Wastewater treatment
A 10 m3 BioCircuit Reactor was utilized for wastewater treatment. Wastewater from an anaerobic pond was introduced into the BCS and operated at three different flow rates (1.0, 1.5, and 2.0 L/min) for a duration of 6 months. Weekly sampling of influent and effluent was conducted, and chemical analysis was performed. Additionally, voltage and power consumption were recorded every 5 minutes via an Internet of Things (IoT) program.
Analysis and calculations
Current (I) and power (P) was calculated as Eq. (9) and (10);
I = V/R (9)
P = V2/R (10),
where V is a cell voltage (V), R is an external resistance (Ω). Volumetric current or power is calculated by dividing the current or power by an anode volume.
The COD, sulfate and sulfide concentrations were analyzed using standard method. A complete block design (CBD) and Duncan test were used for statistical analysis. Denaturing gradient gel electrophoresis (DGGE) genetic analysis was used to identify microbial communities. All analyses were performed in SPSS version 24.0 (IBM, United States). Values mentioned in the tables and text are averages ± one sample standard deviation.
Results and discussion
Isolation, microscopic screening, motility, and identification
A total of 78 bacterial strains were isolated from the rubber wastewater sludge obtained from the first-sampling site. Among these strains, only 8 exhibited the characteristic group-forming shape. These strains, denoted as A7, A13, A16, A21, A26, A36, A40, and A52, are depicted in Figure 2.
Figure 2. The biofilm-forming shape bacterial strains isolated from the rubber wastewater sludge.
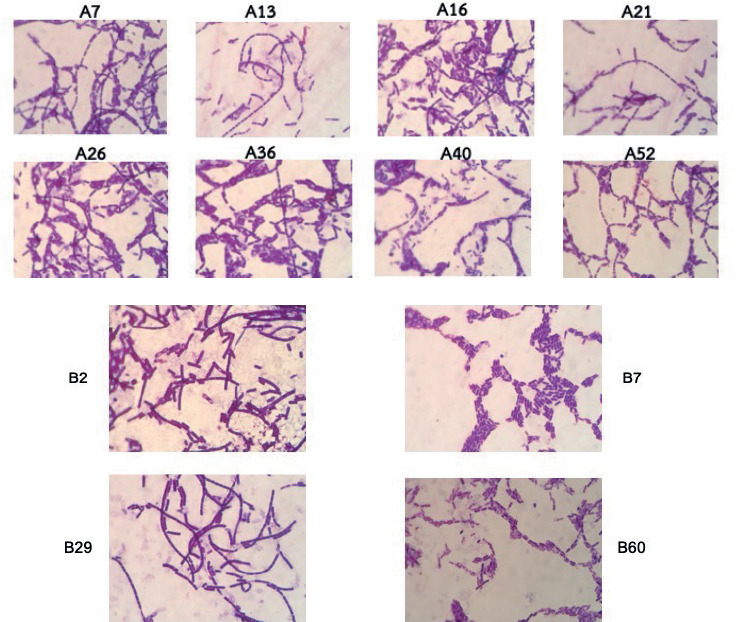
This finding highlights the diversity of bacterial species present in the rubber wastewater sludge and underscores the selective nature of the group-forming shape trait. Identification of these strains and further characterization of their properties will provide valuable insights into their potential applications, particularly in the context of wastewater treatment and bioremediation. The motility of these group-forming bacteria is of particular interest, as it can influence their ability to colonize surfaces and participate in biofilm formation, thereby affecting their efficacy in various environmental processes. Future studies will focus on elucidating the metabolic capabilities and ecological roles of these bacterial strains to harness their potential for sustainable biotechnological applications.
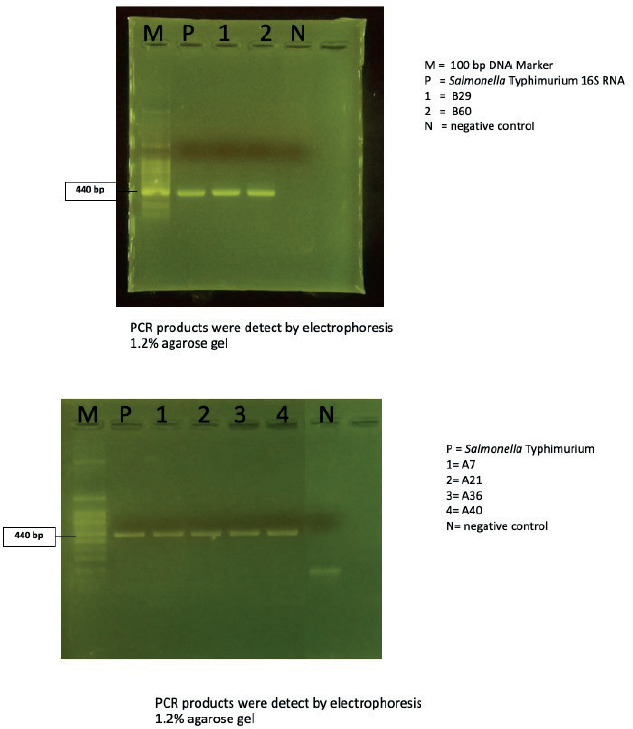
Four of bacterial strains showed the motility ability (strains A7, A21, A36 and A40). In PCR reaction, the 16S rRNA products were amplified. The result revealed the 400-500 base-pairs were achieved from the PCR reaction (Figure 3).
Figure 3. The PCR products of 16S rRNA gene of group-forming shape-forming bacterial strains.
The BLAST results revealed that strains A7, A21, A36, and A40 shared similarities with Bacillus tequilensis, Bacillus sp., Ferribacterium limneticum, and Bacillus weihenstephanensis, respectively. The phylogenetic tree illustrating these relationships is shown in Figure 4. From the rubber wastewater sludge of the second-sampling site, a total of 61 strains were isolated, with only 4 strains exhibiting the group-forming shape, namely strains B2, B7, B29, and B60, as depicted in Figure 2. Among these strains, two (B29 and B60) demonstrated motility ability.
Figure 4. The phylogenetic tree of the group-forming shape bacteria.
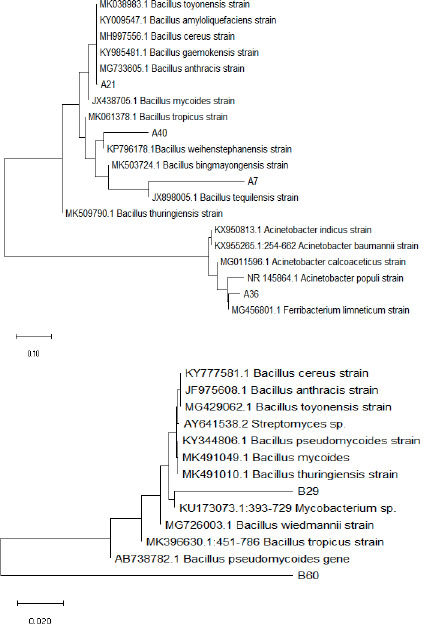
In the PCR reaction, amplification of the 16S rRNA gene resulted in products ranging from 400 to 500 base pairs, as depicted in Figure 3. BLAST analysis indicated that strain B29 exhibited 87% identity with Mycobacterium sp., while strain B60 showed a distinct sequence compared to those in the GenBank database. The phylogenetic tree representing these findings is displayed in Figure 4.
The electricity generation properties of these strains were also assessed. Bacillus sp., as described by N.A. Logan and De Vos (2009), encompasses various species with diverse applications, including enzyme production and bioremediation. Cummings et al. (1999) highlighted the significance of Ferribacterium limneticum within the phylum Bacteroidetes, particularly in freshwater environments, due to its role in biogeochemical cycles and bioremediation.
Furthermore, Martin and Huq (2007) discussed Bacillus weihenstephanensis, emphasizing its applications in antimicrobial compound production and its potential in various industries. Alvarez and Steinbüchel (2002) provided insights into Mycobacterium sp., noting its diverse roles in biodegradation and bioremediation, highlighting its importance in environmental cleanup efforts. These references collectively underscore the diverse characteristics and applications of the bacterial strains identified in the study.
Following the cultivation of each group-forming shape in potato dextrose broth for three days, the half-cell potential and electricity generation properties were systematically evaluated using an Ag/ AgCl reference electrode and microbial fuel cell (MFC), as outlined by Logan et al. (2004). In the MFC testing procedure, cultures of each group-forming shape were introduced into the anode compartment, and the voltage generated by each culture was meticulously recorded using a data logger program at intervals of 1000 milliseconds (Table 1).
Table 1. Electrochemical potential (V) (Ag/AgCl reference electrode) and power potential (MFC test) (V) of each biofilm-forming bacteria.
| Code of bacteria | Species | Electrochemical potential (V) (Ag/AgCl reference electrode) | Power potential across 1 kΩ (MFC test) (V) |
|---|---|---|---|
| A07 | Bacillus tequilensis | 0.409 | 0.172 |
| A21 | Bacillus anthracis, Bacillus gaemokensis, Bacillus cereus, Bacillus amyloliquefaciens, Bacillus toyonensis | 0.468 | 0.241 |
| A36 | Ferribacterium limneticum | 0.418 | 0.128 |
| A40 | Bacillus weihenstephanensis | 0.425 | 0.167 |
| B29 | Mycobacterium sp. | 0.350 | 0.166 |
| B60 | ND | 0.392 | 0.313 |
The sludge obtained from the two sources was combined and inoculated into a 1 m3 reactor, constituting 20% of the working volume. This mixture was allowed to settle for a period of 30 days. Subsequently, the system was initiated at a flow rate of 0.5 L/min for an additional 30 days in open circuit mode. Following this initial phase, the system was fully operationalized at three different flow rates (1.0, 1.5, and 2.0 L/min) in closed circuit mode, with connection to a 10kΩ external resistance.
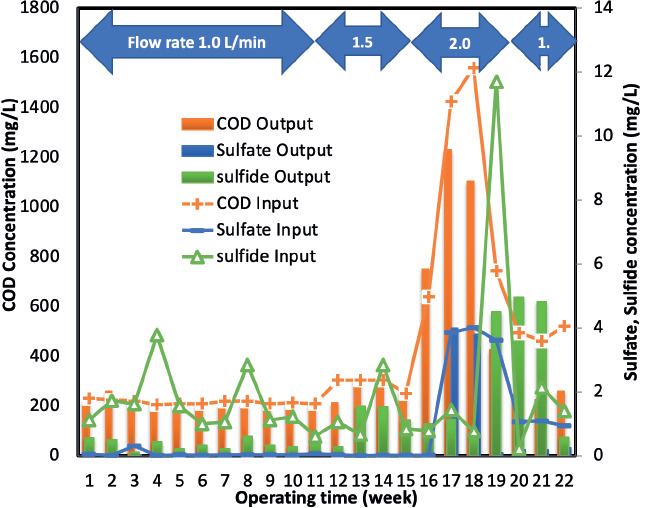
The results obtained demonstrated that the optimal flow rate of 1.5 L/min yielded a maximum voltage output of 1.2 V, as illustrated in Figure 5. Additionally, this flow rate was associated with high wastewater treatment efficiency, as depicted in Figure 6. This phenomenon may be attributed to the enhanced generation of electrons through biodegradation and bioelectrochemical reactions, which were maximized at this specific flow rate.
Figure 5. Power potential across 10kΩ external resistance of the BioCircuit at various flow rates.
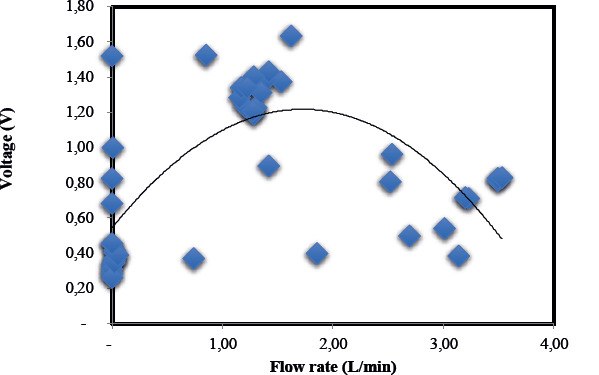
Figure 6. Wastewater treatment efficiency of the BioCircuit at various flow rates.
Conclusions
The operational power consumption of the BioCircuit System (BCS) was calculated to be 0.257 kWh per cubic meter of treated wastewater. This represents a significant reduction of 88.90% in carbon footprint compared to traditional methods such as aerated lagoon treatment, translating to a reduction of 50.94 kg CO2 emissions per cubic meter of treated wastewater, or a substantial annual reduction of 183,384 kg CO2 for a 10 m3 plant.
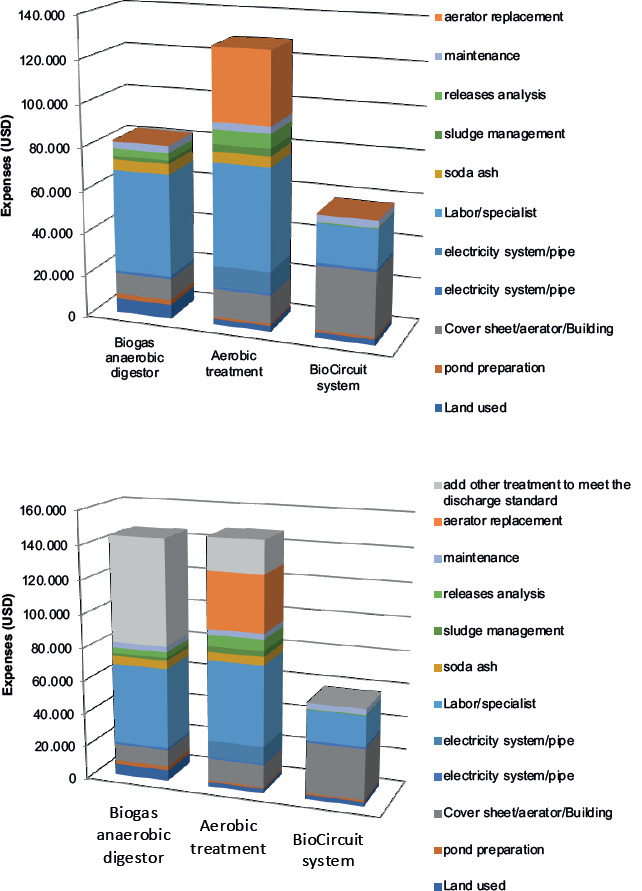
Furthermore, the feasibility of implementing the BioCircuit System for wastewater treatment in practical applications was assessed in comparison to anaerobic and aerobic treatment methods. The analysis revealed a noteworthy cost-saving advantage, with approximately half of the expenses typically incurred over a 5-year operational period (amounting to 6,500 USD per cubic meter) being conserved (Figure 7).
Figure 7. The investment cost and operation expenses (USD/5 y) of 5 m3 BioCircuit wastewater treatment.
These compelling economic benefits, coupled with the demonstrated efficiency and effectiveness of the BCS when integrated with group-forming shape bacteria communities, underscore its potential for widespread adoption across various industrial wastewater treatment applications.