Introduction
Nanotechnology combines various areas of science. Many nanomaterial types have been introduced up to now, for various applications. Among them, magnetic nanoparticles are of great interest for researchers in biomedical science, catalysis and environmental remediation (Elliott & Zhang, 2001; Govan & Gunko, 2014; Gupta & Gupta, 2005). The iron oxide (II, III) based magnetic nanoparticles are the most promising among the other magnetic nanoparticles (Antone et al., 2019). In particular, Fe3O4 magnetite nanoparticles have recently attracted great attention due to their different features compared to individual nanoparticles and took place greatly in materials science, biochemistry and diagnostics (Nguyen et al., 2021). Magnetic iron oxide nanoclusters have unique properties, because they combine the properties of individual nanoparticles and exhibit collective behavior through interactions between individual nanoparticles (Kostopoulou & Lappas, 2015). In addition to this, magnetic iron oxide nanoparticles (Fe3O4 NPs) offer unique properties such as abundance, low toxicity and chemical and photochemical stability, making them an attractive material for usage in certain applications (Naseem & Durrani, 2021). The behaviors of these nanoclusters can be enhanced by the capping molecules leading to novel functional materials. Numerous studies have been conducted on the passivation of iron oxide nanoparticles using capping agents to control the morphology and shape (Mbuyazi & Ajibade, 2023). Besides the synthesis of homogeneous core magnetic Fe3O4 NPs, advanced nanoarchitectures such as core–shell and composites have been highlighted in biomedical applications (Zhang et al., 2006). For this aim, silver nanoparticles (AgNPs) were used to functionalize the Fe3O4 NPs as inorganic supports constructing the core-shell structure. Immobilization of Ag NPs on the surface of Fe3O4 NPs has been shown to limit the aggregation of nanostructure, which renders the applicability of the structures in related applications (Kumari et al., 2019).
Glutathione (GSH) is a water-soluble tripeptide composed of the amino acids and known to function directly or indirectly in many important biological phenomena. GSH plays an important role in plants, mammals, fungi and some prokaryotic organisms as a detoxification agent (Townsend et al., 2003). Several in vivo studies have reported the efficiency of this kind of antioxidant substances against viral infections. It was observed that the introduction of GSH reduced some viral infections (Palamara et al., 1996). Although the antiviral activity of antioxidant substances has been demonstrated clearly, the big handicap is that some molecules, such as GSH, are not freely transported into most cells. For this reason, we aimed to combine the target drug with the drug carrier system, allowing easier access to the membranes of many cell types. Magnetic iron oxide and silver nanoparticles as combined or individually have been used in many applications, but GSH coated derivatives of two different nanoparticles will be the first example of in vivo examination. Incidences of emerging infections have posed serious public health. Although infectious outbreaks can be controlled within a few years, new dangers of infectious diseases will likely depend on evolving and re-emerging infections. Moreover, viral infections caused by the development of antiviral drug-resistant strains have posed a serious threat globally, resulting in high mortality and economic burden, especially in immunocompromised patients (Parvez & Parveen, 2017). Therefore, it is important to develop new antiviral drugs with a different mode of action than those currently in use.
In our study, glutathione (GSH) functionalized Fe3O4 NPs (Fe3O4-GSH) and glutathione functionalized AgNPs immobilized on Fe3O4 NPs to obtain Ag@Fe3O4-GSH as core-shell nanoparticles were obtained. Later, the antiviral activities of the two nanostructures and GSH were examined against different DNA (Human Herpes Simplex Type 1 and Human Adenovirus Type 5) and RNA (Human Poliovirus Type 1, and Bovine Coronavirus) viruses.
Materials and methods
Chemicals and instruments
Iron acetylacetonate, benzyl ether, oleic acid, oleylamine, 1,2-Hexadecanediol, ethanol, acetone, chloroform (CHCl3), methanol, glutathione (GSH), Ag(ac)3, tetramethylammonium hydroxide (TMAOH) were purchased from Sigma–Aldrich. Ultra-pure water was from MilliQ water. Size determination and zeta potential using dynamic light scattering (DLS) was performed using a Nano-ZS instrument (Malvern Instruments Ltd, Malvern, UK). Electronic spectra were recorded on a PerkinElmer LAMBDA 25 Series UV−vis spectrophotometer with a quartz cell of 1 cm. The morphologies of the Fe3O4-GSH and Ag@Fe3O4-GSH core shell nanoparticles were assessed using transmission electron microscope (TEM) JEOL JEM-2100Plus 200kV. X-ray diffraction (XRD) data were collected over the 2θ = 10–100° range using a Bruker D-8 Advance diffractometer, under Cu Kα radiation (λ = 1.5405 Å, 40kV - 40 mA ).
Synthesis
The Fe3O4-GSH was synthesized as outlined in the literature with a slight modification, in two steps (Robinson et al., 2010). In the first step, iron acetylacetonate (0.71 g, 2 mmol) was dissolved in benzyl ether (20 mL) with oleic acid (2 mL, 6 mmol) and oleylamine (2 mL, 4 mmol) under N2 with vigorous stirring. 1,2-Hexadecanediol (2.58 g, 10 mmol) was added into the solution and heated under reflux for 2 hours, then cooled to room temperature. The resultant magnetite NPs were separated by precipitation with ethanol (10 mL) and cleaned with acetone. Finally, the product was dried using a vacuum oven at 50 °C. In the second step, Fe3O4 NPs (0.5 g) suspension in a mixed liquid of CHCl3 (15 mL) and methanol (5 mL) was sonicated for 20 minutes. Then, glutathione (0.4 g) dissolved in water (5 mL) was added to the solution, and sonication method was applied to the final colloidal solution for 2 hours. Then, the mixture was precipitated with ethanol and washed few times with ethanol and water, dried in oven at 50 °C, yielding the final magnetic Fe3O4-GSH NPs.
The Ag@Fe3O4-GSH was synthesized as reported in the literature with slight modification in three steps (Bankole & Nyokong, 2016; Robinson et al., 2010). After applying the same first step for the preparation of Fe3O4 -GSH, the prepared bare Fe3O4 NPs (0.5 g), Ag(ac)3 (0.83 g, 2.2 mmol), 1,2-hexadecanediol (3.1 g, 12 mmol), oleic acid (0.5 mL, 1.5 mmol) and oleylamine (3 mL, 6 mmol) were added to benzyl ether (30 mL) under N2 flow with vigorous stirring. The reaction solution was heated to 180–190 °C and held at this temperature for 1.5 hours. After cooling to room temperature, ethanol was added into the solution and the magnetic material collected by precipitation. The precipitated product was washed with ethanol and re-dispersed in 15 mL of TMAOH solution. Glutathione (0.125 g) in 2 mL degassed water was added to the NP solution and sonicated for 40 minutes to allow for ligand exchange to take place. Water soluble glutathione functionalized Ag@Fe3O4 NPs (Ag@Fe3O4-GSH) were precipitated and washed with ethanol and water to remove unbounded GSH from the surface of the NPs. Finally, the obtained nanoparticles were dried under vacuum at 50 °C.
Cell culture and viruses
Green monkey kidney cells (Vero-CCL81, ATCC) were used for analyses of human herpes simplex type 1 (MacIntyre, Zeptometrix), Adenovirus type 5 (Adenoid 75-, ATCC), Poliovirus type 1, (LSc 2ab, ATCC). Madin Darby Bovine Kidney Cells (MDBK-NBL-1, ATCC) were used for Bovine coronavirus (Mebus, ATCC) analysis. All cell lines and viruses were obtained from the Genetic and Bioengineering Department of Yeditepe University, (Istanbul, Turkey).
Cytotoxicity assay and antiviral activity
Cytotoxicity assay and antiviral activity studies were based on Spearman-Kärber method (Ramakrishnan, 2016) and EN14476 European standard method. Briefly, for cytotoxicity assay, Vero cells were seeded 4x104 cell/well in 96 well plate with DMEM-H and incubated at 37 ºC, 5% CO2 incubator for 24 hours. Then, cells were treated with Log10 serial dilution of Ag@Fe3O4-GSH, Fe3O4-GSH and GSH and incubated at 37 ºC 5% CO2 incubator for 24 hours to determine maximum nontoxic dose (MNTD). Vero cells were seeded based on the same procedure for monolayer formation in each well to determine antiviral activity. Viruses were diluted based on Log10 serial dilution with virus medium (1% PSA, MEM, Gibco) and the material MNTD applied on them for 15 minutes. Then, old media was removed from cell monolayer and washed with PBS three times. The mixture of the virus and nanoparticles and GSH were transferred on to cells and incubated for 72 hours. At the end of the incubation period, the cytopathic effect (CPE) due to the viral infection was evaluated under an inverted microscope. Results were calculated with the following formulas; where X k refers to highest dilution dose, d: represents the difference between dilutions, n represents the number of wells per dilution, r: (-) is the sum of the answers, Mv is the value of antiviral activity, Lg (Vc) refers to the logarithmic average of virus control biological replicates and Lg (Va) is the logarithmic average of experimental biological replicates.
M= Xk + d x [0.5 – (1/n) x (r)] [1]
Mv = Lg(Vc) − Lg(Va) [2]
Results and discussion
The Fe3O4-GSH NPs were synthesized in benzyl ether as organic solvent with oleic acid and oleylamine, resulting in surface coated with hydrophobic ligands. This was followed by the ligand exchange procedure to obtain the coated with GSH (Robinson et al., 2010). The Ag@Fe3O4-GSH NPs were obtained with a two step procedure, including the formation of the Fe3O4 NPs first, followed by the reduction of a silver salt in order to add a layer of Ag on the iron oxide NPs, which was dissolved in benzyl ether. This core-shell nanoparticles were then functionalized with GSH (Bankole & Nyokong, 2016; Robinson et al., 2010). Ultraviolet-visible (UV-vis) absorption spectroscopy and X-ray diffraction (XRD) were used to detect the presence of Ag in the NPs and transmission electron microscopy (TEM) was employed to determine the morphology of the Fe3O4-GSH NPs and Ag coated Fe3O4 NPs. Dynamic light scattering (DLS) was utilized to demonstrate the size of each nanoparticle and zeta potential measurement was used to see the charge of the each nanoparticle.
TEM micrographs
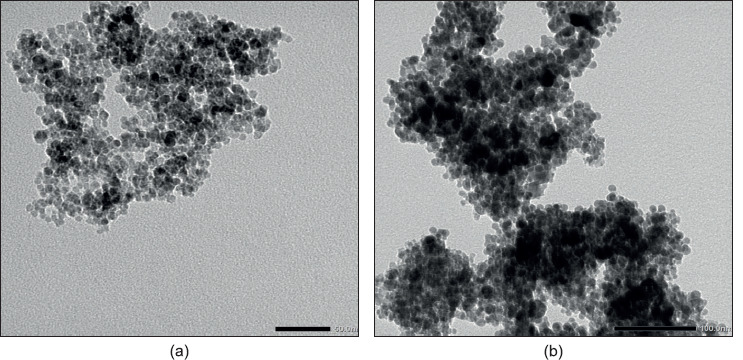
The transmission electron microscope (TEM) was applied to determine the morphologies and shape of the Fe3O4-GSH and Ag@Fe3O4-GSH, as shown in Figure 1. The TEM micrograph for Fe3O4-GSH showed almost mono dispersed, non-aggregated, quasi-spherical shape particles. Upon capping of Fe3O4 with silver to form Ag@Fe3O4-GSH NPs, in addition to small, transparent and evenly dispersed Fe3O4 grains, some large, dark-colored Ag NP grains were obtained, and further aggregation was observed as compared to the pure Fe3O4-GSH NPs.
Figure 1. TEM images of a) Fe3O4-GSH, b) Ag@Fe3O4-GSH.
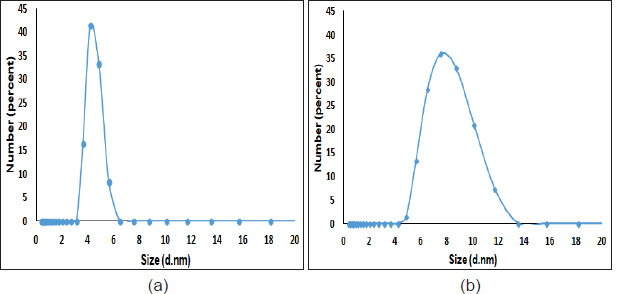
Dynamic light scattering (DLS) spectra and zeta potential
DLS was used for the determination of particle size for both type of the nanoparticles (Figure 2). The average size determined by DLS was 4.48 nm for Fe3O4-GSH. The size increased to 7.98 nm for Ag@Fe3O4-GSH, which indicated the successful surface coating of silver for Fe3O4 nanoparticles.
Figure 2. DLS graph showing average particle size of a) Fe3O4-GSH, b) Ag@Fe3O4-GSH.
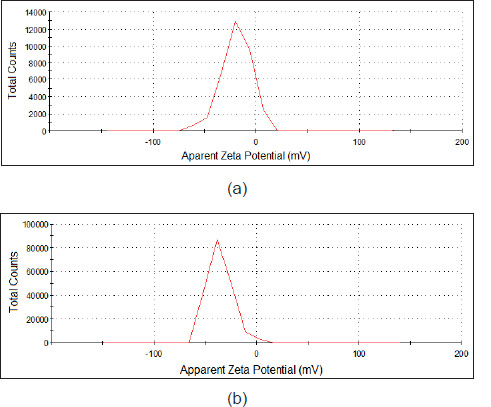
Zeta potential measurements allow us to evaluate the colloidal stability in solution, as well as NPs surface charge. Surface charges of the studied nanoparticles are shown in the zeta potential graphs (Figure 3). The zeta potential value was determined as -19 mV for Fe3O4-GSH and -35.7 mV for Ag@ Fe3O4-GSH in ethanol suspension. The huge negative surface charge of the Ag@Fe3O4-GSH shows its higher stability due to the repulsive force among particles (Khashan et al., 2017).
Figure 3. Zeta potential of a) Fe3O4-GSH, b) Ag@Fe3O4-GSH dispersed in ethanol.
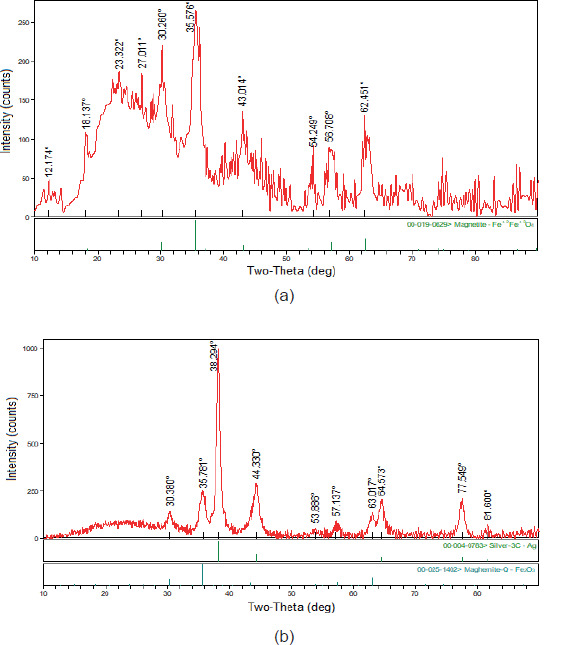
X-ray diffraction (XRD)
XRD was employed for investigation of crystallinity of the prepared NPs and XRD patterns of Fe3O4-GSH nanoparticles and Ag@Fe3O4-GSH core-shell nanoparticles, as shown in Figure 4. The observed characteristic diffraction peaks for Fe3O4 (2 θ= 30.2, 35.5, 43.0, 54.2, 56.7 and 62.4o) can be assigned to diffraction of Fe3O4 crystal with an inverse spinal structure (Malekia et al., 2020). The XRD diffraction patterns of the Ag@Fe3O4-GSH nanoparticles showed well-defined crystalline peaks at 2 θ= 30.3, 35.7, 38.3, 44.3, 53.8, 57.1, 63.01, 64.5, 77.5 and 81.6o. The peaks that are characteristic of silver at 38.3, 44.3, 64.5, 77.5 and 81.6o corresponding to the face centered-cubic structure of metallic silver were not observed for Fe3O4-GSH NPs due to the presence of the Ag shell on the magnetic NPs and the other peaks at 2 θ=30.3, 35.7, 53.8, 57.1 and 63.01o were similar crystalline peaks, which are shifted compared to the Fe3O4-GSH NPs crystalline planes.
Figure 4. X-ray diffraction patterns of a) Fe3O4-GSH, b) Ag@Fe3O4-GSH.
Optical properties
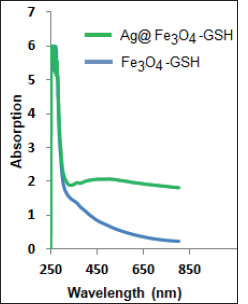
The UV–vis absorption spectrum was applied to characterize the synthesized nanoparticles (Figure 5). The optical absorption spectra of the Fe3O4-GSH exhibit absorption peak at around 250 nm. This peak is related to the electrons moving from the oxygen atom to the d-metal orbital (Ramesh et al., 2017). The large bump in absorption spectrum of Ag@Fe3O4-GSH NPs at around 480 nm was due to resonant excitation of surface plasmons (SPR) of the Ag surface (Xu et al., 2006).
Figure 5. UV–Visible absorption spectrum of Fe3O4-GSH NPs and Ag@Fe3O4-GSH NPs.
Antiviral activity
Antiviral activity analysis can be performed for screening and evaluating viral entry inhibition, viral genome replication blocking and viral maturation inhibition (Rumlová & Ruml, 2017). In this research, antiviral activities of Ag@Fe3O4-GSH, Fe3O4-GSH and GSH were evaluated for viral entry inhibition. Ultimately, significant inhibition was observed with Ag@Fe3O4-GSH against Poliovirus (4 Log), Adenovirus (3 Log), and HSV-1 (2 Log). GSH exhibited remarkable antiviral effects against Bovine coronavirus (3 Log) and showed a log reduction of 1 Log against HSV-1 and Poliovirus. Fe3O4-GSH demonstrated a reduction of 1 Log, particularly against RNA viruses, such as Poliovirus and Bovine coronavirus (Table 1). Among these, only Ag@Fe3O4-GSH nanoparticles have an antiviral effect against all type of viruses at least 99%, while their activity against Bovine coronaviruses was 90%. This situation demonstrates that GSH and Fe3O4 molecules, when combined with silver to form Ag@Fe3O4-GSH nanoparticles, exhibit a synergistic effect against human DNA and RNA viruses. However, synergistic effect was not observed against bovine coronavirus. This can be attributed to host differences, variations in viral membrane proteins, and diversity in host receptors (Teissier et al., 2011). Studies have shown that a deficiency in glutathione, a potent antioxidant, can lead to the accumulation of reactive oxygen species (ROS) in cells, potentially resulting in immune suppression and faster progression of infections (Khanfari & Al Qaroot, 2020). The obtained results regarding glutathione support this information. This is because glutathione alone demonstrates a reduction of 1 log against HSV-1 and Poliovirus, and a reduction of 3 logs against bovine coronavirus, particularly enhancing its efficacy against human viruses in nanoparticle form. Given that bovine coronavirus belongs to the same family as SARS-CoV-2 and is considered as a model virus for SARS-CoV-2 in the literature (Naqvi et al., 2020), it is believed that glutathione, either alone or in combination with human host cells, may also exhibit antiviral activity against SARS-CoV-2.
Table 1. In vitro antiviral activity of the studied samples.
| Compounds | Log reduction | |||
|---|---|---|---|---|
| HSV-1 | Adenovirus | Poliovirus | BCoV | |
| Ag@Fe3O4-GSH | 2 | 3 | 4 | 1 |
| Fe3O4-GSH | - | - | 1 | 1 |
| GSH | 1 | - | 1 | 3 |
Although it has been revealed that GSH depletion plays a role in a wide variety of viral infections (Beck et al., 2000) and nanoparticles made of silver can be effective antiviral agents against varieties such as HIV-1 (Sun et al., 2005), hepatitis B virus (Lu et al., 2008), herpes simplex virus type 1 (Baram-Pinto et al., 2009), influenza virus (Papp et al., 2010), the antiviral activity of GSH coated silver nanoparticles as shell around the magnetic nanoparticle core have not been reported.
Conclusions
The antiviral activity of iron oxide nanoparticles against HSV-1, poliovirus, adenovirus and bovine coronavirus were investigated. In order to see the capping agent (GSH) effect over the studied viruses, GSH was also investigated. Among the prepared magnetic nanoparticles, the nanoparticles containing a magnetic core and a silver shell (Ag@Fe3O4-GSH) exhibited remarkable activity than the bare magnetic nanoparticles (Fe3O4-GSH).