Introduction
Aging is a multifaceted process characterized by disruptions in the intricate balance of biological systems. This imbalance manifests through alterations in tissue, organ, and intracellular biochemical dynamics. Notably, cellular aging ensues alongside the functional decline of mitochondria, perpetuating a cascade of physiological changes (Palombo et al., 1997; Qadir et al., 2020). Advancing age correlates with a heightened susceptibility to age-related ailments, including type 2 diabetes, hypertension, and dyslipidemia (Giansanti et al., 1996; van den Berg et al., 2009). Dyslipidemia, a condition marked by lipid imbalance, serves as a precursor to various cardiovascular diseases, notably atherosclerosis (Bae & Moon, 2024). Lipids, essential biomolecules, intricately participate in cellular membrane structure, energy reservoirs, signal transduction, and synthesis pathways. Dysregulation in lipid homeostasis can also precipitate neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease (Schwartz et al., 2013).
Peroxisome proliferator-activated receptors (PPARs), integral members of the nuclear receptor superfamily, exert profound influence on various disease pathologies, including diabetes, cancer, insulin resistance, hyperlipidemia, and inflammation (Balakumar et al., 2009; Chi et al., 2021; Gu et al., 2014). Notably, dysregulation and insufficiency of this receptor activity emerge as pivotal contributors to this pathological state. PPAR-α orchestrates the transcriptional regulation of proteins involved in fatty acid synthesis and modulates lipoprotein metabolism. Pharmacological activation of PPAR-α stimulates fatty acid oxidation and induces alterations in lipoprotein metabolism (Terra et al., 2008). Fibrates, known as PPAR-α agonists, occupy a crucial role in dyslipidemia management. These agents, exemplified by GW590735, effectively modulate plasma triacylglycerol (TAG) and lipoprotein levels by elevating high-density lipoprotein cholesterol (HDL-C) while concurrently reducing low-density lipoprotein (LDL-C), TAG, and very low-density lipoprotein cholesterol levels (Okopien et al., 2017).
Animal models of dyslipidemia, often induced through diverse methodologies, provide invaluable insights into disease pathogenesis. For instance, high fructose intake in animals mirrors dyslipidemia, characterized by diminished HDL-C, elevated LDL-C, TAG, and total cholesterol (TC) levels in serum samples (Jensen et al., 2021). Similarly, in a D-galactose-induced aging model, administration of 100 mg/kg D-galactose demonstrated exacerbation of dyslipidemia and oxidative stress upon silencing Apo lipoprotein E, a key regulator of lipid metabolism and cholesterol homeostasis (Hakimizadeh et al., 2023).
With advancing age, perturbations in intracellular mechanisms become evident, such as compromised fluidity in the lipid-rich cell membrane, impacting permeability. Furthermore, disruption in the antioxidant-oxidative stress equilibrium accompanying aging precipitates heightened lipid peroxidation, culminating in cell membrane integrity compromise via oxygen free radical-mediated attacks on lipids. The resultant formation of peroxide radicals accentuates lipid degradation within the cell membrane, perpetuating cellular dysfunction (Spiteller, 2002). Understanding the intricate interplay between dyslipidemia, aging, and intracellular mechanisms elucidates novel therapeutic avenues and underscores the imperative of targeted interventions in mitigating age-associated dyslipidemia and its attendant complications (Shao et al., 2011).
Oleuropein, a phenolic compound abundant in olive fruits, exhibits diverse therapeutic properties, including anticancer, antioxidant, anti-inflammatory, antimicrobial, and antiviral effects (Campolo et al., 2013). Remarkably, administration of oleuropein mitigated TAG levels in mice through PPAR-α activation and PPAR-γ inhibition (Ahamad et al., 2019). To evaluate oleuropein’s protective effects against the detrimental manifestations of aging, D-galactose was used to induce the process of aging. Evaluation of oleuropein using this approach is the distinctive feature of our study. D-galactose is a well-known agent used to accelerate and induce the process of aging and its manifestations bringing about decreased antioxidant capability, disturbed lipid parameters and decreased PPAR expression as well as behavioral manifestations (Hakimizadeh et al., 2022; Zhou et al., 2017). Three groups were designed as control (negative control), aged and oleuropein pretreated and aged (Ruan et al., 2013), and the plasma levels of TAG, HDL, LDL, TC, and liver PPAR-α expression were analyzed in all three groups. By scrutinizing dyslipidemia and its associated parameters in light of existing literature, the impact of oleuropein, an antioxidant, on these indices will be assessed. Furthermore, this investigation offers insights into the molecular mechanisms underlying PPAR-α agonists in dyslipidemia, alongside the therapeutic potential of phytochemicals in managing dyslipidemia in vivo.
Materials and methods
Chemicals and reagents
Olive leaves hydro alcoholic extract was collected from Sankara Brain and Biotechnology Research Center (Istanbul, Türkiye) and identified by Professor. Dr. Ihsan Kara. According to the supplier, the extracts were 20% pure oleuropein and the other 80% consisted of various other phytochemicals of olive leaf extracts. D-galactose was acquired from Sigma-Aldrich Co. (Merck KGaA, Darmstadt, Germany) and dissolved in physiological saline at a concentration of 20 mg/mL. All other chemical compounds and kits utilized in this research were also purchased from Sigma-Aldrich Co. (Merck KGaA, Darmstadt, Germany).
Preparation of experimental groups
This study is a continuation of a previously conducted animal experiment with the ethics committee decision taken on 13.08.2020. 24 male Wistar albino rats (220-250g) were provided by the animal lab of Uskudar University, Istanbul, Turkey. The rats were housed in regulated conditions at 22 °C following a 12-hour light and 12-hour dark cycle, within standard cages, ensuring unrestricted availability of both water and food. In this experiment, three groups of 8 were designed. Group 1 was set as the control group and physiological saline (5 ml/Kg) was injected intraperitoneally. Group 2 was given 150 mg/kg/five days a week Dgalactose via subcutaneous injection for 6 weeks (Hakimizadeh et al., 2022; Ruan et al., 2013). In the 3rd group (OLE+D-galactose), oleuropein was administered via intraperitoneal injection at a dose of 200 mg/kg for 30 days. Subsequently, a subcutaneous injection of 150 mg/kg D-galactose was administered for 6 weeks, starting a day or two after the oleuropein treatment.
Tissue homogenization
The liver tissues of the animals were homogenized with cold PBS at a ratio of 1:9. The resulting homogenate was centrifuged at + 4°C, 5000xg for 5 minutes. Following this, the supernatant was taken and stored at -80°C until its utilization for the experiment.
Measurement of plasma TAG, LDL-C, HDL-C levels
Upon collecting the blood of the test subjects, the plasma was centrifuged at 1000xg for 15 minutes at 2-8 °C. The supernatant of the tubes was collected and stored at -80 °C until the experimental stage. TC, LDL-C, HDL-C and TAG levels were measured using the spectrophotometric method with the help of their respective kits (Albers et al., 1978; Allain et al., 1974; Friedewald et al., 1972; McGowan et al., 1983).
Measurement of PPAR-α levels of liver tissue
PPAR-α levels of homogenized liver tissues were analyzed with the ‘Rat PPAR-α E-EL-R0725’ Elisa kit. Analysis of the samples was measured in a spectrophotometer at 450 nm.
Statistical analysis
Statistical analyses were performed using Graph pad software version 9.5.1. The values were shown as mean ± standard deviation. All data were tested for normal distribution using the Kolmogorov Smirnov and Shapiro-Wilk tests and also by graphical quantile-quantile plots. Data conforming to normal distribution were analyzed using one-way ANOVA and post hoc Tukey’s test for multiple comparison while for data that was not normally distributed, Kruskal-Wallis and Dunn’s test was employed for multiple comparison. P<0.05 was considered statistically significant.
Results and discussion
Dyslipidemia, characterized by abnormal levels of plasma TAG, LDL-C, and HDL-C, is intricately linked with irregularities and deficiencies in PPAR-α activity (Terra et al., 2008). The experimental design anticipates the manifestation of PPAR-α imbalance and consequent dyslipidemia formation in rats subjected to D-galactose-induced aging. Analysis of the experiment’s outcomes reveals elevated plasma TAG and LDL-C levels and decreased HDL-C levels in D-galactose-treated rats compared to the control group. However, administration of 200 mg/kg of our oleuropein sample as pretreatment prior to the aging process of the rats attenuated TAG and LDL-C levels, suggesting a potential therapeutic role for oleuropein in age-related dyslipidemia. Consistent with existing literature, D-galactose-induced aging is associated with diminished PPAR-α expression in the liver, a trend corroborated by our findings (Zhou et al., 2017).
Plasma lipid parameters
The rat plasma levels of TAG, TC, HDL-C and LDL-C were assessed using their respective kits. Detailed results obtained from the plasma lipid parameter analyses are presented in Table 1. When comparing TAG levels, both D-galactose aged and oleuropein pretreated aged groups exhibited statistically significant increases compared to the control group. However, the increases in the TAG of the OLE+Dgalactose group was significantly lesser than the aged group. In the comparison of TC levels, the pretreated aged group exhibited statistically significant lower levels, whereas the aged group exhibited statistically significant higher levels when compared with the control group. Similar results were also observed in the LDL-C levels. The HDL-C levels were significantly lower in both D-galactose and OLE+D-galactose groups compared to the control group, with no significant difference observed between the two treated groups.
Table 1. Lipid parameter levels of control, D-galactose, OLE+D-galactose groups. Pairs with similar superscript alphabets were not significantly different at (p<0.05).
| Groups | TAG (mg/dL) | TC (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) |
|---|---|---|---|---|
| Control | 52 ± 0.12 | 65.4 ± 0.1 | 29.2 ± 0.12 | 36.2 ± 0.16 |
| D-galactose | 60.2 ± 0.19 | 67.5 ± 0.15 | 28.61 ± 0.14a | 38.8 ± 0.12 |
| OLE+D-galactose | 55.92 ± 1.2 | 63.9 ± 0.12 | 28.75 ± 0.12a | 35.15 ± 0.17 |
Several natural sources with lipid profile-modulating properties have been identified. These include plants such as Lippia citriodora, Lippia triphylla, Grifola frondose, Rosmarinus officinalis Linn and Curcuma longa (curcumin), which have demonstrated lipid-lowering abilities through mechanisms such as antioxidant activity and PPAR modulation (Roghani-Shahraki et al., 2021). The list of such natural sources continues to expand with ongoing research. A recent study by Kar et al. (2022), the protective effects of pomegranate juice against aluminum-induced hepato-nephrotoxicity and the subsequent increase in lipid profiles were evaluated. The study found that the pomegranate juice effectively lowered LDL and TC levels.
Oleuropein has been shown to exhibit remarkable protective effects against oxidative damage in tissues, coupled with a significant reduction in serum levels of TC and TAG. This protective mechanism was elucidated in a study by Andreadou et al. (2006), shedding light on OLE’s role as a potent antioxidant and lipid-lowering agent. In a separate study conducted by Malliou et al. (2018), the administration of oleuropein at a dosage of 100 mg/kg mixed with the food of mice for a duration of 6 weeks yielded noteworthy reductions in TAG underscoring the therapeutic potential of oleuropein in mitigating dyslipidemia and metabolic disorders. Furthermore, Ahmadvand et al. (2016) evaluated the lipid profile of rats with induced nephrotoxicity treated with oleuropein. Similar to our findings, they also observed protective effects of oleuropein with reduced levels of TAG, TC and LDL. They also witnessed an increase in HDLC which we herein did not observe against d-galactose-induced aging.
Liver PPAR-alpha levels
Liver tissues were homogenized together with PBS at a ratio of 1:9 and PPAR-α levels were assessed using the PPAR-α E-EL-R0725’ Elisa kit. Samples were run in duplicate. As illustrated in Figure 1, the animal group that underwent the aging process through D-galactose treatment yielded significantly lower liver PPAR-α levels in comparison with the control group. Conversely, OLE+D-galactose group exhibited results akin to those of the control group, significantly surpassing the aged group.
Figure 1. PPAR-α measurements of the control, aged (D-galactose) and oleuropein pretreated and aged (OLE+D-galactose) groups. Difference between bar pairs marked with * (p<0.05) and ** (p<0.001) are significant (n=8).
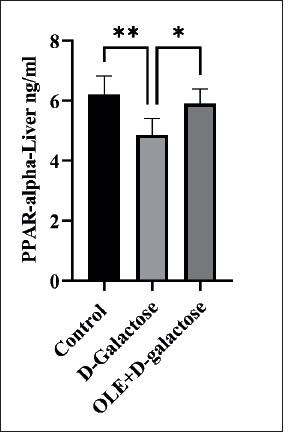
Oleuropein’s effect on PPAR-α activation aligns with its ability to reduce TAG levels, as PPAR-α plays a pivotal role in lipid and lipoprotein metabolism. Understanding the molecular mechanisms underlying PPARs and their interplay with lipoprotein metabolism emphasizes the potential of PPAR agonists as pharmacological agents for dyslipidemia management. Synthetic PPAR ligands such as fibrates, statins, and hypolipidemic drugs modulate plasma lipid levels by reducing TAG and LDL-C levels while augmenting HDL-C levels. Notably, oleuropein emerges as a natural PPAR-α ligand, as evidenced by its ability to enhance PPAR-α and retinoid X receptors (RXR) homodimerization. Furthermore, oleuropein upregulates LDL receptor expression in the liver and modulates genes involved in TAG synthesis, uptake, transport, metabolism, and elimination, akin to fibrates, albeit with potentially fewer associated side effects (Malliou et al., 2018). In our study, the observed elevation in PPAR-α levels in the oleuropein treated group further substantiates oleuropein’s efficacy in mitigating dyslipidemia. This could indicate that oleuropein acts as both an activating ligand and a facilitator in upregulating PPAR-α, countering the detrimental effects of aging.
In another study regarding oleuropein and PPAR activities, it was determined that oleuropein concentrations between 10-400 μM had an inhibitory effect solely on the transcriptional activity of PPARγ, without impacting other PPARs in vitro. The anti-adipogenic effects observed in their study was attributed to this property of oleuropein (Svobodova et al., 2014). In research conducted by Feng et al. (2016), bavachinin, a natural compound extracted from malaytea scurfpea fruit, demonstrated Pan-PPAR agonistic properties and contributed to glucose and lipid reduction in diet-induced obese mice. Combining these findings with our research, it is evident that oleuropein, at high doses, is capable in inhibiting PPAR-γ while simultaneously activating PPAR-α yielding anti-adipogenic and lipid profile lowering effects. This would be more favorable to Pan PPAR agonistic compounds such as bavachinin as continuous activation of PPAR-γ has been linked with weight gain, heart failure and bone loss.
The study was limited by the number of animals provided, preventing the designation of negative dyslipidemia controls or positive controls treated with fibrates. Consequently, this limitation positions this study as a preliminary investigation rather than a comprehensive analysis. Nevertheless, these findings highlight the potential of oleuropein as a promising therapeutic agent for managing metabolic disorders as well as a preventive agent against the process of aging, offering valuable insights into its underlying mechanisms of action and paving the way for further exploration in clinical settings.
Conclusions
In light of our study, we conducted an examination of PPAR-α expression levels, as well as levels of LDL-C, HDL-C, and TAG, pivotal components in the dyslipidemia mechanism within our aging model. By elucidating the effects of our oleuropein sample on liver PPAR-α levels and plasma lipid profiles, we have demonstrated its ability to regulate lipid metabolism and improve dyslipidemia in aging individuals. The profound influence of PPAR agonists on lipid and lipoprotein metabolism underscores their significance as promising targets for drug development. Understanding the intricate molecular mechanisms underlying PPARs and their interplay with lipoprotein metabolism emphasizes the therapeutic potential of PPAR agonists in regulating plasma lipid levels and ameliorating dyslipidemia. To the best of our knowledge, apart from the research by Malliou et al. (2018), no other investigation demonstrated meaningful effects of oleuropein on PPAR-α prior to our study. Further research on the effects of oleuropein on PPAR-α and its subsequent outcomes is warranted. Given the diverse array of natural and synthetic agonists employed in cardiovascular disease management and lipid profile regulation, our study underscores the importance of further investigations into aging, particularly at the genetic level, with emphasis on oleuropein as a natural PPAR-α agonist. Moreover, our study contributes to the growing body of evidence supporting the use of natural compounds as potential interventions for age-related health conditions.