Introduction
Many microencapsulation technologies have been developed to accommodate the increasing use of probiotics in functional foods. In the food industry, spray-drying is the most common method to encapsulate probiotic bacteria. This technique presents the advantages of low cost, reproducibility, and rapidity in incorporating probiotics into dairy products (Gardiner et al., 2002; Sharma et al., 2022). On the other hand, the major disadvantage of this technology is the use of high air temperatures that can cause a decrease in bacterial survival after incorporating the bacteria into the encapsulating material through the spray drying process (Tripathi and Giri, 2014; Bommasamudram et al., 2022). In this regard, many encapsulating materials have been tested to produce innovative matrices maintaining the viability of probiotic bacteria at a functional rate during the spray-drying process (Tirta et al., 2023), the subsequent storage (Gullifa et al., 2023), and finally, their passage through the human gastrointestinal tract. In these instances, the viability of the bacteria is evaluated by the direct culturing on agar, also known as colony plate count method (Guerin et al., 2017). Although culture-dependent methods are always used, they do not always provide reliable information about the viability of cells since they cannot detect viable but non-cultivable (VBNC) bacteria. The VBNC state is induced naturally in cells by environmental stresses such as high temperature and drying (Zhao et al., 2017), as exemplified by the case of spray drying. Bacterial cells in this state may not grow on the standard bacteriological media on which they previously developed colonies. However, they are still alive and can show metabolic activity (Oliver, 2005). Bacterial physiological indicators should be more sensitive to estimate bacterial survival. Among various methods using the level of enzymatic activity to monitor microbial population density, assays based on biotransformations of tetrazolium salts (TS) have gained much popularity. This test principle is based on the enzymatic reduction of lightly colored TS into an intense purple-blue colored formazan, which can be quantified spectrophotometrically. For a long time, the use of TS reduction has been limited to eukaryotic cell research. In contrast, the application of this procedure for viability estimation of microbial cells following TS reduction by bacteria is still poorly understood (Tachon et al., 2009). One of the most common examples of TS used in bioassays is 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT). MTT has been applied in cell proliferation assays (Tsukatani et al., 2008), multidrug-resistant bacteria detection (Montoro et al., 2005), biofilm formation evaluation (Brambilla et al., 2014) and indirect quantification of antibacterial compounds (Wang et al., 2007). MTT produces less background absorbance than WST-5, WST-8, and XTT, making it a more reliable choice (Wang et al., 2010). MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H tetrazolium) assay is often described as a ‘one-step’ MTT assay, since it allows a researcher to add the reagent directly to the cell culture without the addition of DMSO or isopropanol, as needed in the MTT assay. The MTS compound produces a formazan product, which in the presence of phenazine methosulfate (PMS), has a maximal absorbance at 490-500 nm in phosphate-buffered saline. The ability of bacteria to reduce tetrazolium salts depends on the salt type and the bacterial species. For example, (Tsukatani et al., 2008) showed that Gram-positive bacteria generally reduced TS that form water-soluble formazan better than Gram-negative bacteria. Thus, the TS assays must be conducted under correctly optimized conditions for a reliable absorbance value proportional to the number of viable cells (Berridge et al., 2005).
The present work investigated the potential of the MTS-based method for monitoring the viability of encapsulated lactic acid bacteria (Lactobacillus rhamnosus GG and Lactobacillus plantarum 299v) in different matrices after spray-drying. The VBNC state of encapsulated bacteria was estimated through the difference in bacteria enumeration results between the colony plate counting method and the newly optimized MTS assay. The sensitivity of both approaches in assessing bacteria losses and, therefore, the microencapsulation efficiency under different conditions was also evaluated.
Materials and methods
Culture media and growth conditions
The encapsulation by spray-drying was performed on mesophilic lactic acid bacteria (LAB) reference strains L. rhamnosus GG (ATCC 53103) and L. plantarum 299v. L. rhamnosus GG were purchased from the American Type Culture Collection (Lot: 70006824), while L. plantarum 299v was recovered from a commercial probiotic supplement. The identity and the purity of the strain have been confirmed by MALDI TOF (Bruker Daltonics, Bremen, Germany). Man–Rogosa–Sharpe (MRS) agar medium (Acumedia, USA) propagated the bacteria. MRS broth (Acumedia, USA) was used as a pre-culture medium for the inoculum preparation. L. rhamnosus GG and L. plantarum 299v were cultured at 37 °C and 5% CO2 for 48 hours on MRS agar and then overnight in MRS broth under shaking.
Preparation of microencapsulation materials and feeding solution
Whey protein isolate (WP) (Davisco, USA), maltodextrin (MD) (Sigma Aldrich, Germany), and trehalose (Tr) (Cargıll, Germany) were used as materials for LAB microencapsulation. WP solution was prepared by rehydration of whey protein isolate at 15% (m/v) in 100 mL of sterile distilled water (SDW). Rehydration was carried out as described by Guerin et al. (2017). The solution was stirred briefly for 2 hours at room temperature (25 °C). After rehydration, WP solutions were denatured by heating at 78 °C for 10 minutes and cooled at 4 °C. MD and Tr solutions were prepared by rehydration of the powder at 15% (m/v) in 100 mL of SDW before being autoclaved at 121 °C for 15 minutes. The feeding solution was prepared by mixing the encapsulation material solution (100 mL) with an overnight culture (taken at the beginning of the stationary phase) of the LAB strains to a final concentration of 1011 cfu/mL.
Spray-drying conditions
LAB strains were encapsulated in different encapsulation materials using a spray-dryer (Bakon B15, Turkey) equipped with a 0.7 mm nozzle under the following conditions: pump 3 mL/min, aspirator 80% and compressor 11 L/min. Different inlet temperatures (InletT) were applied: 105 °C and 115 °C. The outlet temperatures (OutletT) obtained were 68-69 °C and 73-75 °C, respectively (Guerin et al., 2017).
Bacterial cultivability
Conventional colony plate counting was used to study the effect of the spray-drying process and storage conditions on the cultivability of the LAB cells. One gram of dry samples was rehydrated in 10 mL of PBS solution for 5 minutes at room temperature under stirring. The homogenized solution was serially diluted with PBS (1X) (Sigma, Germany) and plated on MRS agar. The colony-forming units were determined after 48 hours of incubation at 37 °C and 5% CO2.
MTS assay optimization
The correlation between the MTS absorbance at OD490 and the number of living cells from fresh cultures of LAB were established in initial experiments to be used for bacterial VBNC state assessment after spray drying and during storage. For this purpose, CellTiter 96® AQueous MTS Reagent Powder (Promega, USA) was applied according to the manufacturer’s instructions. Briefly, ten milliliters of an overnight bacterial culture of L. rhamnosus GG or L. plantarum 299v were aliquoted into ten Eppendorf tubes and centrifuged at 11,000 g (Sigma 3-18KS, Germany) for 15 min at 4 °C to discard the supernatant. Ten-fold and two-fold serial dilutions of the bacterial pellet were suspended in the MTS master mix containing PBS (1X) + glucose (4.5 g/L) and MTS 10 % (v/v). It is important to note that three passages of the bacterial suspension through an aseptic syringe needle are needed. One milliliter of each bacterial suspension was used to determine colony-forming units (CFU) after plating on MRS agar and incubation at 37 °C and 5% CO2 for 48 hours. The remaining suspension was incubated under the same conditions for 5 hours. After every 20 minutes, 1 mL of the MTS bacterial solution was centrifuged, the pellet was discarded, and the supernatant was added to a 96-well plate (100 µL/well) for optical density analysis. The optical density was recorded by a microplate scanning spectrophotometer (Bio-Rad xMark, USA). The correlation of the MTS optical density units at OD490
(ODU490) to colony-forming units of fresh cultures enumerated on MRS agar was established by a regression analysis using Microsoft Excel (2013). Killed bacteria, generated by incubation in 70% isopropyl alcohol for 30 min., were used as a negative control.
VBNC state assessment
The entrapped bacteria were released from microcapsules according to the method of Semyonov et al. (2011) with some modifications. One gram of spray-dried powders was rehydrated in 10 mL of PBS (1X) (Sigma-Adrich, UK) for 5 minutes under stirring at room temperature. As described above, the bacterial pellet obtained by centrifugation was washed twice and re-suspended in the MTS master mix. After 3.5 hours of incubation in the dark at 37 °C and 5% CO2, bacterial solutions were centrifuged at 11,000 g for 15 minutes at 4 °C. The optical density of the supernatant was then measured at 490 nm. Killed bacteria obtained from encapsulated LAB dissolved into 70% isopropyl alcohol for 30 minutes (mixed every 15 minutes) were washed with PBS (1X) and recovered for use as a negative control specific for each encapsulation material. A bacteria-free master mix was used as a general negative control.
The amount of viable entrapped LAB is determined according to the previously established correlation between ODU490 and the fresh cultivable inoculum (C) of LAB (cfu/g). The VBNC state is expressed in cfu/g, and the percentage of VBNC level (%) was calculated as follows:
VBNC= V-C [1]
VBNC (%)=log10(VBNC)x[100/(log10I)] [2]
Where “V” is the viable encapsulated LAB (cfu/g) obtained by MTS assay, “C” is the culturable encapsulated LAB (cfu/g) enumerated on MRS agar by the conventional spread plate technique, and “I” is the initial LAB inoculum (cfu/g) before the spray drying process.
Evaluation of the microencapsulation
The microencapsulation process was evaluated according to the bacterial losses after microencapsulation by the two methods: i/ the conventional colony plate counting technique (MEC) and ii/ the MTS assay optimized in this study (MER). MEC and MER were calculated as follows:
MEC (%)=100- [100×(C/I)], [3]
MER (%)=100- [100×(V/I)], [4]
Where “C” is the culturable microencapsulated LAB (log10cfu/g) on MRS agar, “I” is the initial LAB inoculum (log10cfu/g) before the spray-drying process, and “V” is the viable microencapsulated LAB (log10cfu/g) determined by the MTS assay.
Statistical analysis
All the experiments were conducted in triplicate (n = 3). Data were subjected to analysis of variance (ANOVA) using SPSS software (version 20). The significance of mean differences was determined using Duncan’s test, and responses were judged significant at the 5% level (P ≤ 0.05).
Results and discussion
Measuring the survival of probiotic organisms under harsh conditions encountered during food processing or through the gastrointestinal system is a significant challenge for researchers and producers. The viability of the starter cultures is traditionally quantified by the colony plate counting method, which evaluates the ability of cells to grow in an appropriate medium. Hence, the population of cultivable bacteria is determined. However, under stress conditions and different industrial processes used to produce dried biomass, many bacterial species enter a physiologically VBNC state (Guerin et al., 2017; Semyonov et al., 2011). In the VBNC state, although they are alive, bacteria cannot develop colonies on standard bacteriological media on which they used to grow (Oliver, 2005). Therefore, cell viability evaluation based on the ability to form colonies may lead to an underestimation of viable cells, as in the case of microencapsulation. Additionally, culture-dependent methods lack the resolving power to provide real-time results, and bacteria may occur in chains and/or clumps, resulting in an underestimation of true bacterial counts (Doherty et al., 2010; Guerin et al., 2017). Thus, more sensitive and rapid methods have been developed to monitor probiotic survival, such as quantitative real-time PCR (Gueimonde et al. 2004), fluorescent in situ hybridization (FISH), and flow cytometry (Breeuwer and Abee, 2005; Lahtinen et al., 2006). However, the industrial adoption failure of these methods results from their lack of cost-efficiency and scope for the extension of product applications (Lahtinen et al., 2006). In this context, a simple colorimetric method, based on the bacterial ability to reduce MTS, was optimized to assess the level of the VBNC state within encapsulated LAB under different conditions and evaluate the efficiency of the microencapsulation process.
Optimization of the MTS assay
Incubation time: The kinetics of MTS reduction by L. rhamnosus GG and L. plantarum 299v cells were determined with different inoculum concentrations (obtained from two-fold serial dilutions), as shown in Figure 1. There was a gradual increase in the absorbance at OD490 and a plateau after almost 3-4 hours for the two LAB strains.
Figure 1. Kinetics of MTS reduction by overnight fresh cultures of (a) L. rhamnosus GG; and (b) L. plantarum 299v, at different cells concentrations: inoculum 1 (▲), inoculum 2 (●), and inoculum 3 (■). Bacterial cells were incubated with MTS and absorbance was read at OD490 each 20 min. Each point is the mean of three replicates.
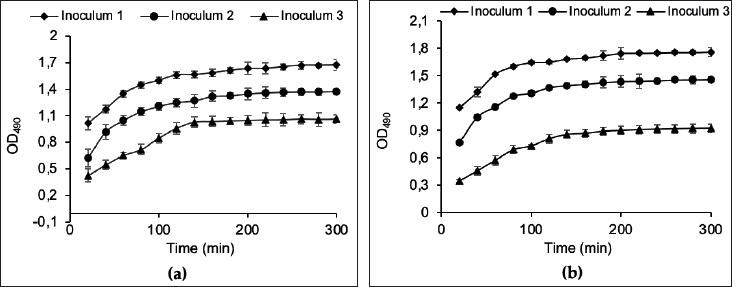
Only a few uses of tetrazolium salts have been reported for lactic acid bacteria (LAB), probably because the acid produced by LAB inhibits tetrazolium salt reduction (Tachon et al., 2009). In this respect, the ability of L. rhamnosus G.G. and L. plantarum 299v to reduce MTS was first verified. We chose 3.5 hours for the standard procedure. Our results agree with the observations of previous works reporting that 3 to 4 hours of incubation with tri-phenyl tetrazolium chloride (TTC) is adequate for developing a measurable color intensity in a log-phase bacterial culture (Tengerdy et al., 1967; Oh and Hong, 2022).
Correlation between ODU490 and CFU: As reported previously, the color intensity of the reduced tetrazolium salt, formed by the reducing activity of bacteria, was proportional to the number of actively growing bacteria in the culture (Tengerdy et al., 1967). Therefore, the correlation between MTS absorbance units at OD490 to logarithmic colony-forming units on MRS agar media was performed in the initial experiments. No coloration was observed with dead bacteria in negative controls, which means that MTS reduction is related to viable bacteria only. Linear relationships between the absorbance obtained by the present method and the colony-forming units were obtained with good correlation coefficients: R2r = 0.9833 (±0,0041) and R2p = 0.9892 (±0.0036) for L. rhamnosus GG and L. plantarum 299v, respectively. The CFUs obtained at various MTS reduction absorbance levels were regressed to linear functions and yield using the following equations:
Yr=(2.108 × Xr) – 395648 [5]
Yp= (3.109 × Xp) – 2.108 [6]
Yr and Yp represent the CFUMTS (cfu/100 µL) of L. rhamnosus GG and L. plantarum 299v, respectively, and Xr and Xp represent the MTS absorbance at OD490 units (ODU490) for L. rhamnosus GG and L. plantarum 299v, respectively.
MTS reduction was related to viable bacteria only. No coloration was observed with dead bacteria. Differences in linear relationships between MTS absorbance and viable cell density (Equations 5 and 6) regressed from the two LAB strains show that this correlation is species-specific, as reported previously for many bacterial species (Tachon et al., 2009; Tsukatani et al., 2008). Therefore, the number of actively growing bacteria of a given species should be calculated from its calibration curves. Nevertheless, there was no reliable absorbance for bacterial densities higher than 5x 1011cfu/mL and less than 104 cfu/mL. With high bacterial densities, a discoloration of MTS was observed. This could be because high bacterial densities can transform formazan into colorless derivatives, as observed previously with prolonged bacterial incubation in tetrazolium salt (Stowe et al., 1995; Xu et al., 2023). The MTS assay was not sufficiently sensitive for very low bacterial densities, as reported previously, with several bacterial species and different tetrazolium salts (Tsukatani et al., 2008; Wang et al., 2007). A concentration of 106 cfu/mL in the product at consumption or a daily intake of 108-109 probiotics is often recommended (Tripathi and Giri, 2014). Considering these reference values, our study’s limits of MTS sensitivity levels are not part of evaluating probiotic encapsulation efficiency. Nevertheless, materials used for encapsulation (WP, MD, and Tr) interfered with the MTS and generated a brownish coloration. Several chemicals were reported to cause abiotic TS reduction in cell-free medium (Grela et al., 2018), such as luteolin, quercetin, plant extracts (Peng et al., 2005), enzyme inhibitors (Weyermann et al., 2005), cysteine, and thioglycolate-containing medium (Oren et al., 1987), salts and complexes containing copper (II) (Perez et al., 2017). In this respect, we removed the MRS medium and encapsulation materials before the MTS application. Many colorimetric methods based on the capacity of bacteria to reduce tetrazolium salts have been used as a rapid and indirect alternative for cell growth evaluations (Mshana et al., 1998; Foongladda et al., 2002; Montoro et al., 2005; Wang et al., 2007; Tsukatani et al., 2008; Brambilla et al., 2014). The VBNC state has been recently reported in several LAB strains (Liu et al., 2017; Wang et al., 2020). However, to our knowledge, this is the first optimization of the tetrazolium salt-based method for the survivability assessment of microencapsulated lactic acid bacteria in complex matrices.
Cultivability of LAB throughout spray drying
The percentage of cultivable and VBNC state of L. rhamnosus GG and L. plantarum 299v just after the spray drying process were compared under different OutletT(s) and encapsulation materials (Figure 2).
Figure 2. The effect of spray drying conditions on the cultivability of LAB strains after microencapsulation with different matrix at different outlet temperatures: WP-whey protein, MD-Maltodextrin and Tr-Trehalose. (■) Outlet temperature = 68-69 °C and (□) Outlet temperature = 73-75 °C.
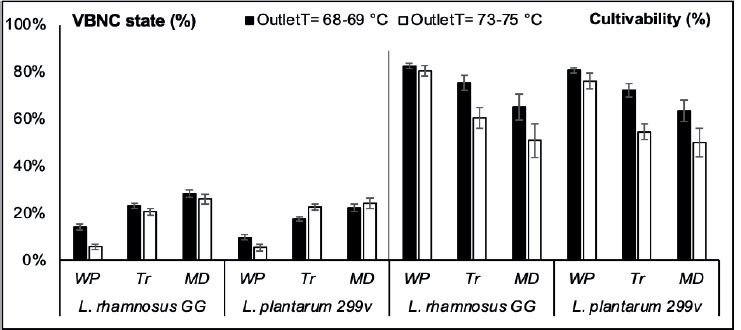
Viabilities of LAB strains, after spray drying under different conditions estimated according to equations 5 and 6, were compared to the colony plate counts on MRS agar. The difference between the two methods revealed the importance of the VBNC state level within the encapsulated LAB. This physiological state was related to different factors related to microencapsulation and storage conditions. L. rhamnosus GG and L. plantarum 299v showed different responses, in the matter of VBNC state level, toward the OutletT increasing under various encapsulation materials.
Compared to L. plantarum 299v, L. rhamnosus GG was a relatively better spray-drying survivor. Heat resistance is a strain-specific trait that has already been reported in previous studies (Gardiner et al., 2002; Goderska and Czarnecki, 2008; Pérez-Chabela et al., 2017; Malmo et al., 2021). Although the loss of cultivability was significantly (P ≤ 0.05) less exhibited by L. plantarum 299v than L. rhamnosus GG under OutletT = 68-69 °C, this was not available under OutletT = 73-75 °C. This is consistent with the fact that the viability of bacteria during spray drying is not only related to thermal tolerance, but also associated with cell damage due to shear stress, as reported previously (Corcoran et al., 2004; Lievense et al., 1994; Kiekens et al., 2019). The lowest levels of VBNC state were recorded when microencapsulation was carried with WP, independently to the used LAB strain or OutletT. Our results show the importance of WP in reducing bacterial cell damage during spray drying. Physical stresses like low or high temperatures and drying induce sublethal damaged bacteria to enter the VBNC state (Zhao et al., 2017; Silva et al., 2012; Arvaniti et al., 2021). Generally, milk proteins are known to offer several advantages in comparison to other widely used biomaterials in the microencapsulation of probiotics (Abd El-Salam and El-Shibiny, 2012; Heidebach et al., 2012; Tavares et al., 2014). This was also confirmed by the plate count method used in this study, given that the highest cultivable population on MRS agar was recorded with WP regardless of the strain type or the OutletT. Moreover, the effect of the spray drying temperature on the cultivability of LAB strains was significant (P ≤ 0.05) only when MD or Tr were used as encapsulation material.
Cultivability of LAB during storage
During storage at room temperature, the percentage of cultivable and VBNC state of spray-dried L. rhamnosus GG and L. plantarum 299v varied differentially according to the used OutletT and encapsulation material (Figure 3).
Figure 3. The evolution of VBNC state and cultivability percentages of encapsulated LAB strains according to spray drying conditions during storage (30 days and 60 days) at room temperatures: WP-whey protein, MD-Maltodextrin and Tr-Trehalose. (■) Outlet temperature = 68-69 °C and (□) Outlet temperature = 73-75 °C.
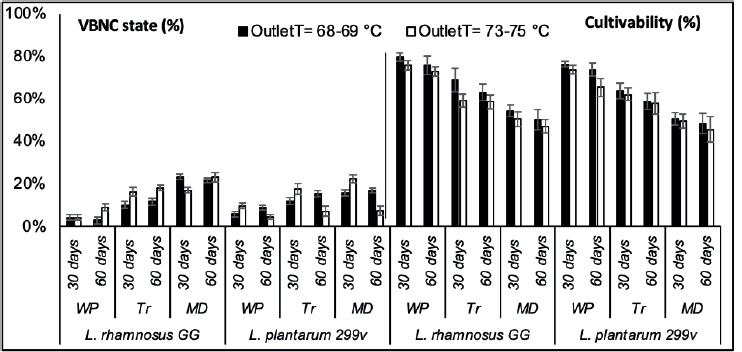
The VBNC state of encapsulated LAB strains was significantly more stable (P ≤ 0.05) during the storage under OutletT (68-69 °C) for both strains encapsulated with different materials. Under higher OutletT (73-75 °C), the VBNC state was more stable with bacteria encapsulated with only WP and Tr. The two LAB strains exhibited the lowest VBNC state in WP powders during storage (i.e., less than 10%). The two LAB strains showed different evolution of their VBNC rate during storage. While the VBNC state of encapsulated L. rhmanosus GG increased with time, under OutletT (73-75 °C), encapsulated L. plantarum 299v showed a decreasing rate of VBNC state. The number of cultivable encapsulated LAB strains significantly decreased after thirty days of storage regardless of the encapsulation material used and the OutletT. The viability of bacteria encapsulated with MD and Tr decreased drastically after thirty days of storage.
Although optimal conditions required to protect probiotics during spray drying and storage are well documented, it is still uncertain that cell damage can effectively predict survival during storage (Chávez and Ledeboer, 2007; Teixeira et al., 1995). In whey powders, LAB strains showed the highest cultivable percentage. Sugar and protein-containing formulations were reported to improve storage stability. Tr was better than MD in maintaining bacterial viability during storage among the two sugar powders used. Silljé et al. (1999) reported that Tr was a storage factor during carbon starvation in Saccharomyces cerevisiae. The changes in cultivability of encapsulated LAB strains were not significant between thirty days and sixteen days of storage except for the L. plantarum strain in WP powders produced at OutletT=73-75 °C. The difference between the amounts of cultivable bacteria of the two LAB species during storage in different powders shows that this response is species-specific, as reported in previous studies (Pérez-Chabela et al., 2017; Corcoran et al., 2004). The impact of higher OutletT on the change of bacterial cultivability during storage was not always significant. This could be explained by the fact that the physicochemical proprieties of powders obtained under different drying temperatures in this study are not sufficiently distinct to affect the storage stability differentially. It was reported that at inlet and outlet temperatures above 100 °C and 60 °C, respectively, the moisture content of the dry powder is below 4%, which is required for safe storage (Chávez and Ledeboer, 2007).
Microencapsulation evaluation
Microencapsulation efficiency was evaluated based on the percentage of dead bacteria after spray drying and storage, according to the two methods as presented in Figure 4. The difference between bacterial losses observed in the two methods was significant, especially after the spray drying process (P < 0.05); the MTS assay showed fewer LAB losses than the direct culture method. Nevertheless, during storage, the effect of the technique used on the recorded bacterial losses was less significant, especially for bacteria encapsulated in WP.
Figure 4. Bacteria losses after microencapsulation according to the MTS assay and the direct culture method, after the spray drying process (After S.P.) and during the storage (after 30 days and 60 days) at room temperature: WPwhey protein, MD-Maltodextrin and Tr-Trehalose. (■) Outlet temperature = 68-69 °C and (□) Outlet temperature = 73-75 °C.
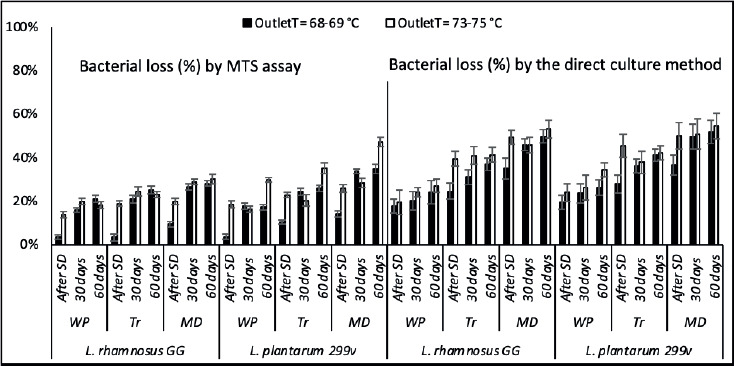
The significance of the OutletT effect on LAB viability was consistently observed with the MTS assay, unlike the direct culture method, which rarely could reveal the impact of higher OutletT on bacterial survivability during storage. The microencapsulation process seems more responsible for the VBNC state induction in LAB strains than starvation during storage.
The high standard deviation values of LAB losses recorded by the direct culture method masked the effect of some factors on the efficiency of the microencapsulation process (especially LAB species, OutletTs, and storage time). In contrast, the MTS assay revealed the differences between the effects of the used OutletTs on the bacterial mortality during the first 30 days of storage and the pronounced sensitivity of strain L. plantarum 299v toward higher Outlet T during the second month of storage. However, the differences between the effects of encapsulation materials on the LAB losses were significant according to both methods. WP exhibited the lowest LAB losses after spray-drying and during storage at room temperature, according to the MTS assay and the direct culture method.
Based on the above observations, the MTS assay optimized in this study allowed the assessment of the VBNC state within encapsulated LAB strains throughout spray drying and storage. It was more accurate in evaluating the encapsulation efficiency than the direct culture method. Besides the high variability of CFU counts on MRS agar within the same treatment, the direct culture method lacks the resolving power to provide real-time results (Lahtinen et al., 2006). Given the bacterial chain fragmentation outcome (Guerin et al., 2017; Doherty et al., 2010) or/and the VBNC state induced after the spray drying process, the use of a culture-based method may result in underestimation of true bacterial accounts as well as the encapsulation efficiency. Moreover, detecting VBNC and injury states within bacteria is relevant in the food industry. Depreciating the product’s safety status may result in a potential risk of foodborne pathogens or reduced shelf life (Silva et al., 2012; Wesche et al., 2009). Sublethal damaged bacterial cells usually can repair their damages under suitable conditions so they can grow again (Espina et al., 2016). Favorable growth conditions with a source of energy and an ideal stoichiometric ratio of carbon to inorganic elements were reported to be capable of reversing the VBNC state (Ayrapetyan and Oliver, 2016; Ramamurthy et al., 2014). Hence, if recovery phenomena occur, the intermediate state between viable and dead cells should be considered when evaluating the probiotic shelf life, particularly in the case of drying technologies. Nevertheless, VBNC bacteria’s fate once they reach their host cells is still unknown.
Conclusions
This study evaluated the efficacy of MTS utilization to assess the VBNC state and the survivability of LAB strains after spray drying and storage. Once optimized, the MTS assay could be a simple, low-cost, real-time, and reliable alternative for the direct culture method to monitor microencapsulated bacterial survival in complex matrices. The VBNC state was detected within bacteria throughout spray drying and during storage by the difference between the expected colony-forming counts estimated by the MTS assay and the enumerated colonies on MRS agar. The accuracy and reliability of the calorimetric method used in our study should be confirmed with an enzymatic or molecular secondary method. The level of the VBNC state within encapsulated bacteria was related to different conditions of the spray drying process. It is still necessary to know the impact of the loss of cultivability on the product’s shelf life and the strain’s efficacy in fulfilling probiotic functions in the gastrointestinal tract.