Introduction
Haloperidol is a butyrophenone derivative antipsychotic agent used in the treatment of schizophrenia and related psychoses (Lee et al., 2007). It was synthesized in 1958 and first marketed in the United States in 1967 (Gerace et al., 2012). While there is a wide choice of antipsychotics, some very expensive, in developed countries, older and less expensive drugs such as haloperidol is still widely used in developing countries (Leucht et al., 2008). Although haloperidol is frequently prescribed and often used as a comparator in clinical trials, both acute and long-term use can cause extrapyramidal side effects such as dystonia, dyskinesia and parkinsonism (Grubor et al., 2020). This drug exerts its therapeutic effect through D2 receptor antagonism, as with other antipsychotic drugs (Tyler et al., 2017). It has a rapid onset of action and an extensive volume of tissue distribution. It is also lipophilic and highly protein bound (Wang et al. 2012). Only 1% of the administered dose of haloperidol is excreted unchanged in the urine because the drug is extensively metabolized in the liver (Gassó et al., 2013). Its metabolism includes conversion to an “inactive metabolite” (50/60%) by glucuronidation, to “reduced haloperidole” (an active metabolite - 23%) by reduction and back oxidation, and to a “pyridium metabolite” (a toxic metabolite 20/30%) by N-dealkylation (Vella-Brincat and Macleod 2004). The CYP2D6 genotype has an important role in the excretion of “haloperidol” and “reduced haloperidol” and may pose a risk of serious adverse effects. In addition, body weight, and smoking may cause changes in pharmacokinetics (Brockmöller et al., 2002). Haloperidol can be used with a variety of medications such as antipsychotics, antidepressants, and benzodiazepines (Shin et al. 2001). Combination antipsychotic therapy is a common practice, but it also has effects such as drug-drug interactions, variability in serum levels, and difficulties in patient adherence to treatment (Lally and MacCabe 2015). Because of this variable pharmacokinetics and narrow therapeutic range, haloperidol therapy requires personalized optimization. For this reason, therapeutic drug monitoring is recommended for haloperidol (Kudo and Ishizaki 1999). The recommended plasma concentration for haloperidol is 1-10 ng/mL, while the alert level is 15 ng/mL (Hiemke et al., 2018). Therapeutic drug monitoring (TDM) helps the clinician reach targeted concentrations and direct dosage to reduce the risk of side effects (Eliasson et al. 2013). TDM is performed by chromatographic and immunoassay methods, but in immunoassays the presence of various other components in the sample can lead to a lack of analytical specificity (Ates et al., 2020). For this reason, reliable, sensitive, high-quality analytical methods by instruments combined with Ultraviolet (UV), Fluorescent (FL), and mass spectrometry (MS) detectors are the main techniques used for TDM (Tuzimski and Petruczynik 2020).
Salt-assisted liquid liquid microextraction (SALLME) is a simple, fast and easily applicable sample preparation technique that uses various salts and water-miscible organic solvents (Gupta et al. 2009). Currently, there is a lack of any SALLME technique in the literature for the determination of haloperidol in human plasma. Therefore, the aim of this study was to develop a rapid, economical and easily applicable LC-MS/MS method for the determination of haloperidol in human plasma using SALLME technique.
Materials and methods
Materials and reagents
Haloperidol, haloperidol-d4, sodium hydroxide, sodium chloride, and organic solvents were procured from Merck (Darmstadt, Germany). Water was purified by a Purelab option-Q water system from Elga (Elga, Lane End, UK).
Stock solutions of haloperidol were prepared separately in methanol at a 1 mg/mL concentration. A working standard solution was prepared by diluting the stock standard solutions with an appropriate volume of methanol. Calibration standards and quality control samples were prepared by spiking the (internal standard) IS and working standard solution into blank plasma. The concentrations of the calibration standard solutions were 1, 2, 4, 6, 8, 10, 13, and 15 ng/mL, while the concentrations of the quality control samples were 1, 3, 7.5,12 ng/mL. All solutions were kept at -20°C when not in use.
Sample preparation
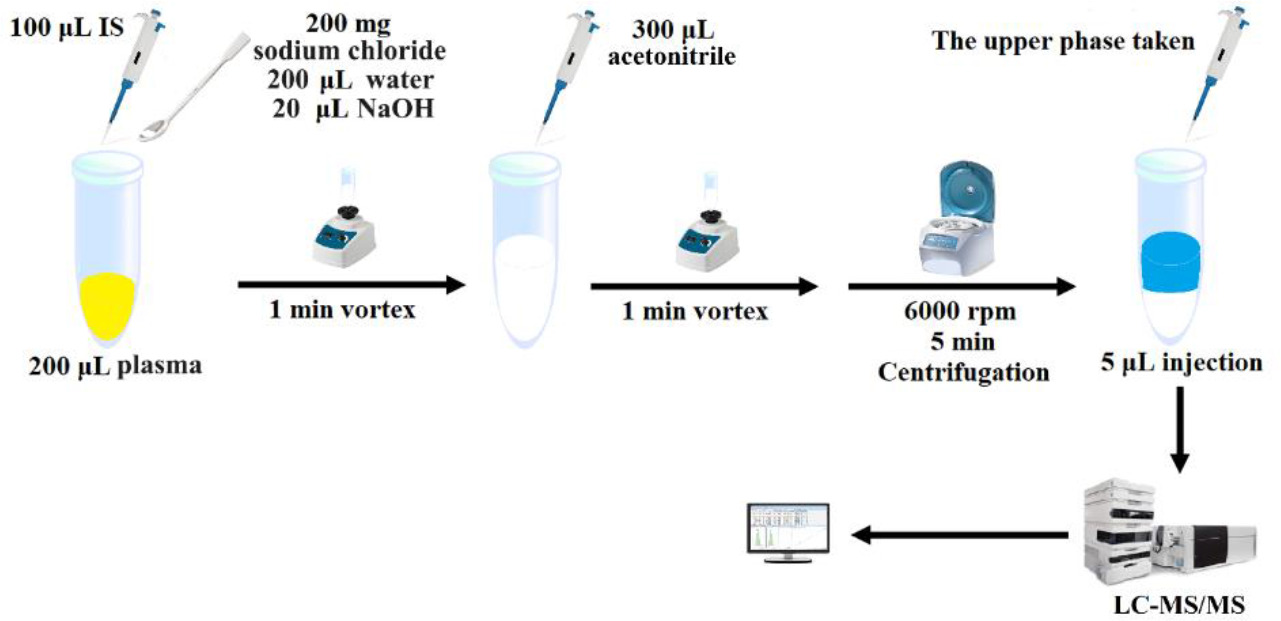
The salt-assisted liquid-liquid microextraction technique was followed for cleaning the plasma samples. The sample preparation procedure is shown in Figure 1.
Figure 1. Sample preparation.
LC-MS/MS analysis
The method was performed on an Agilent 1200 series HPLC system coupled to an Agilent 6460 triple quadrupole tandem mass spectrometry with an InterSustain C18 (3.0 μm, 3.0× 100 mm) column. The column temperature and the injection volume were 30 °C and 5 µL respectively. The isocratic elution was performed using mobile phase A (water with 0.1% formic acid) and mobile phase B (acetonitrile with 0.1% formic acid) (60:40). The total run time was 4 min with flow rate of 0.4 mL/min. The desolvation gas temperature, nebulizer, and desolvation gas flow were set at 300 °C, 45 psi, 11 L/min. The typical ion transitions were 376.2→ 165.1 for haloperidol and m/z 380.2→169.0 for haloperidol-d4. Analytes were detected using electrospray ionization in the positive mode.
Validation of the method
Method validation was performed to evaluate the selectivity, lower limit of quantification (LLOQ), precision, accuracy, dilution integrity, matrix effects, carryover, calibration curve performance, and stability, according to European Medicines Agency (EMA) Bioanalytical Method Validation Guideline (EMA 2011). Selectivity is the ability of the method to separate the analyte from other substances in the matrix. For this purpose, blank plasmas from six different sources were analyzed and examined for any interference. The lower limit of quantification (LLOQ) is the lowest concentration of analyte that can be measured in a sample with acceptable accuracy, reliability, and precision. LLOQ was determined by considering the therapeutic range of the analyte. Accuracy and precision parameters were assessed by preparing quality control samples at 4 different concentrations (LLOQ, low-QC, medium-QC, and high-QC) and analyzing them six times on three different days. Calibration curve performance was evaluated using 8 different calibration concentration levels for the analyte and the calibration curve was defined between the lower limit of quantification and upper limit of quantification (ULOQ). Carryover was evaluated by injecting a blank sample after the standard at the upper limit of quantification. Dilution integrity was assessed by diluting a sample with a higher concentration than the ULOQ sample to within the range of the calibration curve. The stability parameter was evaluated using freshly prepared samples of the analytes and internal standard under 3 different conditions (4 weeks at -20°C, 24 hours in autosampler, 3 times freeze-thaw). In the matrix effect study, low and high QC samples were prepared in the plasma matrix and the solution obtained from six different sources was analyzed. The matrix effect was calculated by proportioning the response values obtained.
Sample analysis
The developed method has been successfully applied to plasma samples from patients on drug therapy.
Results and discussion
Method optimization
In this study, various amounts of sodium chloride salt (150, 200, and 300 mg), organic solution types (acetonitrile and ethyl acetate), and volumes (300, 400, and 500 µL) were tested for phase separation. 200 mg of sodium chloride and 300 µL acetonitrile produced the best yield.
Method validation
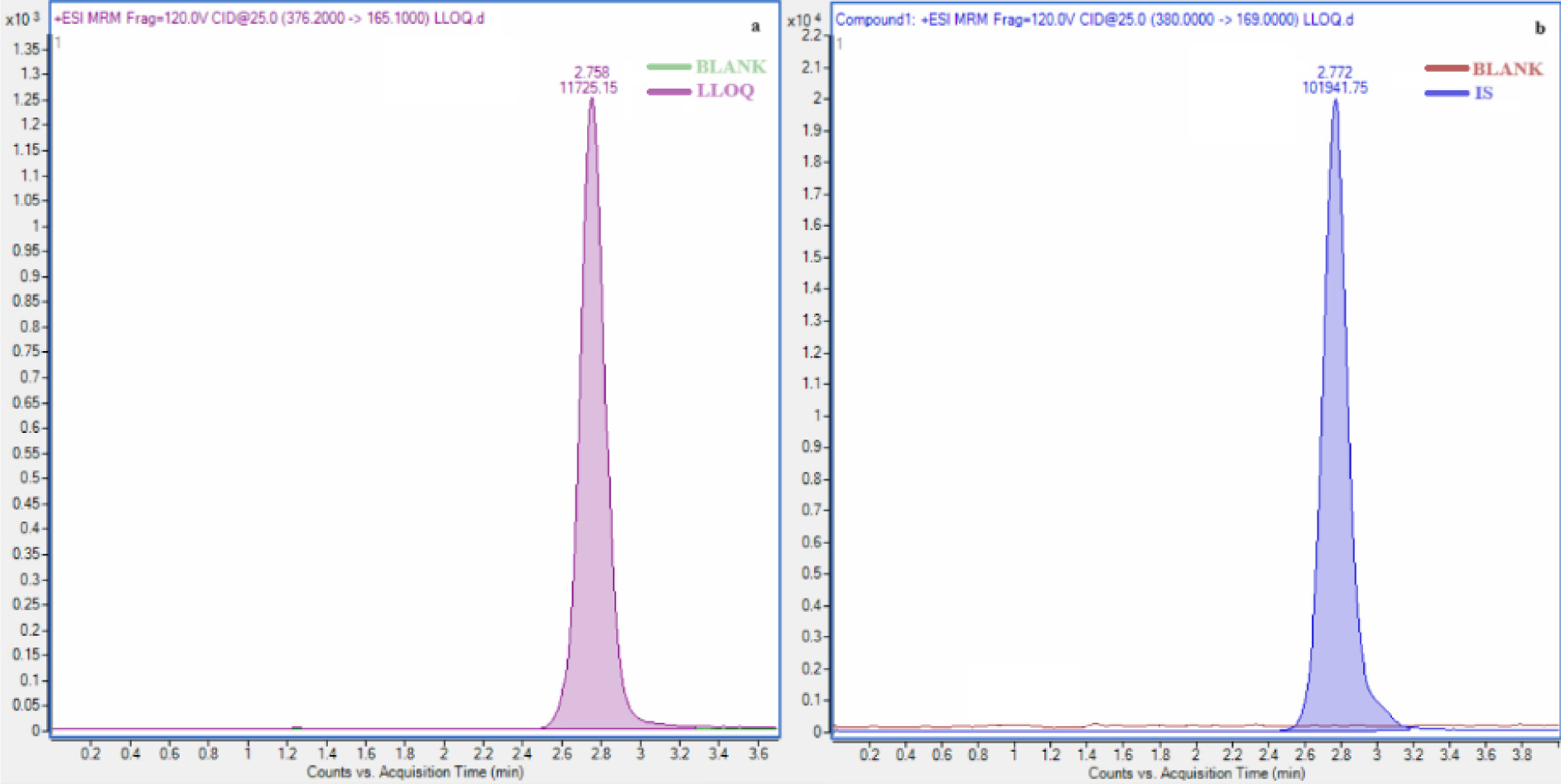
No interference was detected in the retention time of the analytes and internal standard in blank plasma. The retention times for the internal standard and haloperidol (Figure 2). The lower limit of quantification of haloperidol was determined as 1 ng/mL. The calibration curves were validated between 1-15 ng/mL for haloperidol, with correlation coefficients >0.99. No interference was detected in the blank plasma injected after the ULOQ sample.
Figure 2. Chromatograms of the haloperidol (a) and IS (b).
All accuracy and precision values are given in Table 1. It is seen that the results comply with the EMA criteria.
Table 1. Accuracy and precision data for haloperidol.
| QC concentration (ng/mL) | Intra-assay (n=6) | Inter-assay (n=18) | ||
|---|---|---|---|---|
| Accuracy (%) | CV (%) | Accuracy (%) | CV (%) | |
| 1 (LLOQ) | 102.9 | 4.4 | 96.3 | 4.2 |
| 3 (low) | 103.1 | 2.1 | 106.8 | 2.9 |
| 7.5 (medium) | 106.4 | 3.8 | 100.9 | 3.5 |
| 12 (high) | 102.5 | 5.7 | 106.3 | 4.8 |
QC: quality control, LLOQ: lower limit of quantification, CV: coefficient of variation
The accuracy and precision of the diluted samples were 101.7% and 3.4% for haloperidol. The accuracy values of the stability analyses for haloperidol and the internal standard are shown in Table 2. The results comply with EMA acceptance criteria.
Table 2. The stability of haloperidol in plasma.
| QC concentration (ng/mL) | Stability (% Accuracy) | ||
|---|---|---|---|
| 24 hours in the autosampler (n=6) | 4 weeks at −20 °C (n=6) | three freeze-thaw cycles (n=6) | |
| 3 (low) | 104.3 | 102.9 | 108.7 |
| 12 (high) | 105.1 | 103.1 | 106.5 |
QC: quality control
The matrix effect results were within EMA's acceptable ranges (CV, 15%) (Table 3).
Table 3. The matrix effect data of haloperidol.
| QC concentration (ng/mL) | *Normalized matrix effect factor (n=6) | CV (%) | |||||
|---|---|---|---|---|---|---|---|
| 3 (low) | 1.0 | 1.0 | 1.0 | 1.1 | 1.0 | 1.1 | 5.0 |
| 12 (high) | 1.0 | 1.1 | 1.0 | 1.0 | 0.9 | 1.0 | 6.3 |
QC: quality control, CV: coefficient of variation
Application to real sample
Five actual samples were successfully used to test the developed method. Table 4 presents all results.
Table 4. The results of the samples.
| Sample | Age | Dose of haloperidol | Concentration of plasma (ng/mL) |
|---|---|---|---|
| Sample-1 | 19 | 5 mg | 5.5 |
| Sample-2 | 44 | 10 mg | 2.4 |
| Sample-3 | 14 | 10 mg | 9.9 |
| Sample-4 | 46 | 10 mg | 2.3 |
| Sample-5 | 24 | 10 mg | 1.4 |
Method comparison
There are various LC-MS/MS methods in the literature for the determination of haloperidol in plasma and serum. These methods have various sample preparation techniques such as protein precipitation (Juenke et al. 2013, Gradinaru et al., 2014, Domingues et al., 2016, Cao et al. 2020), liquid-liquid extraction (Remane et al., 2011, Montenarh et al., 2016, Degreef et al., 2021), and solid phase extraction (Hempenius et al., 1999, Khelfi et al., 2018). Among these sample preparation techniques, protein precipitation is relatively easy but it has a low recovery. Liquid-liquid extraction requires the use of a lot of organic solvents, while solid-phase extraction is quite expensive compared to these methods. The Sample preparation technique presented in this study is not only easy to apply but also more environmentally friendly as it uses less organic solvents.
Conclusions
The LC-MS/MS method developed using the SALLME technique is simple, accurate, and precise. The values of the European Medicines Agency's bioanalytical method validation parameters are acceptable and therefore, the method is suitable for the therapeutic drug monitoring of haloperidol. Additionally, the SALLME technique can be used as an extraction method in many biotechnological studies.