Introduction
Natural naphthoquinone derivatives have been used in traditional Chinese medicine for centuries and are known to treat disease (Hussain et al., 2007; Cruz et al., 2014; Lopez et al., 2014; Aziz et al., 2008; Masi et al., 2017; Inagaki et al., 2015; Borghese et al., 2017). The Lawsone molecule, an important naphthoquinone derivative, has also been shown to be effective in killing mosquitoes that transmit the Zika virus (Masi et al., 2017; Inagaki et al., 2015). Vitamin K1, which plays an active role in blood clotting, is the most well-known naphthoquinone derivative (Kar et al., 2003; Nishikawa et al., 2015). Naphthoquinones constitute an important subclass of quinones, which are found in nature and from which various derivatives can be obtained synthetically. Plumbagone, juglon, and lawsone are plant-derived naphthoquinones with antibacterial activity against various aerobic and anaerobic organisms. Toxins derived from naphthozarin (5,8-dihydroxy-1,4-naphthoquinone) are produced by Fusarium solani and attack plants, other fungi, and bacteria (Peralta et al., 2015; Riffel et al., 2002). Aliphatic thiols are important starting materials for the synthesis of thiophosphoric acid esters, drugs, and polysulfides and are often used as polymerization modifiers in rubber and plastics production. The most important industrial applications of aromatic thiols are as intermediates in the production of pharmaceuticals, agrochemicals, dyes and pigments, and chemicals for the electronics industry (Vollhardt et al. 2009; Roy et al. 2000). The biological activity properties of amino and thionaphthoquinones are known from the literature (Durán et al., 2023; Kavaliauskas et al., 2022; Liu et al., 2023; López-López et al., 2022; Souza et al., 2023). In this study, novel N,S-substituted naphthoquinone analogues 2, 4, 6, and 8 were synthesized from the reactions of previously known aminonaphthoquinone derivatives 1 (Kalmayer et al. 1991), 3 (Tadashi et al. 1996), 5 (Vanallan et al. 1963), and 7 (Fries et al. 1922) with allyl mercaptan. Synthesized new naphthoquinone analogues (2, 4, 6, and 8) were purified by column chromatography, and their chemical structure was characterized by spectroscopic methods (FT-IR, NMR, MS).
Materials and methods
Chemistry
Infrared (FT-IR) spectra were recorded with the ATR method on a Termo Scientific Nicolet 6700. 1H and 13C NMR spectra were recorded on Spectrometer Varian UNITY INOVA 500 MHz (CDCl3, DMSO-d6 solvent, TMS (δ = 0) internal standard). Mass spectra were obtained on a Shimadzu LC-MS 8030 triple quadrupole mass spectrometer. (Mobile phase A: water (5 mµ ammonium formate), Mobile phase B: acetonitrile (5 mµ ammonium formate)). Products were purified by column chromatography on silica gel (Merck silica gel 60, 0.063-0.2 mm). Melting points were measured on a Buchi SMP20 (B-540) apparatus. Thinlayer chromatography (TLC) was purchased from Merck DC-AlufolienKieselgel 60F254. The solvents used during the reactions and purification of the products were recovered in a Rotavapor Büchi Heatig Bath B-490. Chromatograms were observed using a CAMAG Muttenz-Schweiz 29200 UV lamp. All chemicals used in the reactions were obtained from Merck, Sigma-Aldrich.
Synthesis
General synthesis method. N-substituted naphthoquinone derivative and allyl mercaptan in an equivalent molar ratio were stirred in a 250 mL flask in the presence of 60 mL of dichloromethane and triethylamine for 6 h on a magnetic stirrer at room temperature. The reaction mixture in the flask was extracted with methylene chloride and 200 mL (50 mL x 4) water. The organic phase was dried over sodium sulfate. After recovery of the solvent in the evaporator, the crude products were separated and purified by column chromatography. The chemical structures of the synthesized naphthoquinone analogues were elucidated by spectroscopic methods (NMR, FT-IR, and MS).
2-(allythio)-3-(4-phenylpiperazine-1-yl)naphthalene-1,4-dione (2)
2-(allythio)-3-(4-phenylpiperazine-1-yl)naphthalene-1,4-dione (2) was synthesized from the reaction of 0.5 g (1.417 mmol) 2-Chloro-3-(4-phenylpiperazine-1-yl)naphthalene-1,4-dione (1) and 0.105 g (1.417 mmol) allyl mercaptan according to general synthesis procedure.
2: Red solid. Yield: 0.097 g (% 17.6). FT-IR (ATR):υ (cm-1)= 3200 (CHaromatic), 2963, 2910 (CHaliphatic), 1534, 1501 cm-1 (C=Caromatic), 1638, 1617 (C=O); 1HNMR (500 MHz, CDCl3): δ = 7.9-8.0 (dd, 2H, CHnaphthoquinone), 7.5-7.6 (m, 2H, CHnaphthoquinone), 7.2-7.3 ve 6.9-7.0 (m, 5H, CHaromatic), 5.6-5.8 (m, H, CH=), 4.9-5.0 (m, 2H, =CH2), 3.2-3.7 (m, 6H, N-C H2, S-CH2), 2.4-2.9 (m, 4H, N-CH2); 13C NMR (125 MHz, CDCl3): δ = 182.1-181 (C=O), 154 (Caromatic-N), 145.2 (Cnaphthoquinone-N), 129 (Cnaphthoquinone), 134.5, 132, 131 (Cnaphthoquinone, CHnaphthoquinone), 129.5, 118, 116 (CHaromatic), 37.7 (S-CH2), 117 (=CH2 ally), 133.9 (CH=ally), 52.4 (CH2-N); +ESI: m/z= 391 [M+H]+, m/z =350 [M-(CH2-CH=CH2)]+, C23H22N2O2S, (M=390.5 g/mol).
2-(alylthio)-3-(4-(2-fluorophenyl)piperazine-1-yl)naphthalene-1,4-dione (4) 2-(alylthio)-3-(4-(2-fluorophenyl)piperazine-1-yl)naphthalene-1,4-dione (4) was synthesized from the reaction of 2-chloro-3-(4-(2-fluorophenyl)piperazin-1-yl)naphthalene-1,4-dione (3) (0.6 g, 1.618 mmol) and allyl mercaptan (0.12 g) to general synthesis procedure.
4: Dark red viscos. Yield: 0.179 g (% 27.1). FT-IR (ATR):υ (cm-1)= 3010 (CHaromatic), 2950 (CHaliphatic), 1646 (C=O), 1541, 1500 (C=C); 1HNMR (500 MHz, CDCl3): δ = 7.5-8.0 (m, 4H, CHnaphthoquinone), 6.8-7.0 (m, 4H, CHaromatic), 5.6-5.8 (m, H, CH=), 4.8-5.0 (m, 2H, =CH2), 3.6-3.8 (m, 2H, S-CH2), 3.2-3.5 ve 2.5-3.0 (m, 4H, CH2-N); 13C NMR (125 MHz, CDCl3): δ = 182.2-182.1 (C=O), 154.9 (Cnaphthoquinone-N), 126.8 (Cnaphthoquinone-S), 132.3, 133.0, 134.0 (Cnaphthoquinone, CHnaphthoquinone), 157 (Caromatic-F), 124.8, 119.7, 116.6, 116.4 (Caromatic, CHaromatic), 37.7 (S-CH2), 118.1 (=CH2), 51.7 (CH2-N); +ESI: m/z= 409.15 [M+H]+, m/z= 368.15 [M-(CH2-CH=CH2)]+ , C23H21FN2O2S, (M=408.49 g/mol).
2-(allythio)-3-(4-benzylpiperidine-1-yl)naphthalene-1,4-dione (6)
2-(allythio)-3-(4-benzylpiperidine-1-yl)naphthalene-1,4-dione (6) was synthesized from the reaction of 0.6 g 2-(4-Benzylpiperidine-1-yl)-3-chloronaphthalene-1,4-dione (5) and 0.122 g allyl mercaptan to general synthesis procedure.
6: Dark red viscos. 0.123 g (% 18.6). FTIR (ATR):υ (cm-1)= 3199 (C-Haromatic), 2950, 2905 (C-Haliphatic), 1638, 1618 (C=O); 1HNMR (500 MHz, CDCl3): δ = 7.9-8.0 ve 7.5-7.6 (m, 4H, CHnaphthoquinone), 7.0-7.3 (m, 5H, CHaromatic), 2.0-3.0 ve 1.1-1.4 (m, 10H, 5 CH2), 5.6-5.9 (m, H, CH=), 4.9-5.2 (m, 2H, =CH2), 3.5-3.6 (m, 2H, CH2-S); 13C NMR (125 MHz, CDCl3): δ = 179.2 (C=O), 143.4 (Cnaphthoquinone-N), 132.6, 130.8, 129.8, 128.1, 127.8, 127.2, 125,8 (Cnaphthoquinone, CHnaphthoquinone, CHaromatic), 29.3 (CH2 piperidine), 41.1 (CH2-N), 25.4 (Cpiperidine), 37.7 (CH2-S); +ESI: m/z= 404 [M+H]+, m/z= 331[M-SCH2-CH=CH2]+, C25H25NO2S, (403.54 g/mol).
2-(allylthio)-3-(4-chlorophenylamino)naphthalene-1,4-dione (8)
2-(allylthio)-3-(4-chlorophenylamino)naphthalene-1,4-dione (8) was synthesized from the reaction of 0.4 g (1.54 mmol) 2-(4-Chlorophenylamino)-3-chloronaphthalene-1,4-dione (7) and 0.114 g (1.54 mmol) allyl mercaptan to general synthesis method.
8: Dark red viscos. 0.147 g (% 26.9). FT-IR (ATR):υ (cm-1)= 3319 (NH), 3050 (Caromatic), 2917, 2849 (C-Haliphatic), 1666, 1639 (C=O), 1592, 1551 (C=C); 1HNMR (500 MHz, CDCl3): δ = 7.6-8.1 (m, 4H, CHnaphthoquinone), 6.8-7.3 (m, 4H, CHaromatic), 5.6-5.7 (m, H, CH=), 4.9-5.0 (m, 2H, CH2 allyl), 2.0-3.0 (m, 2H, CH2-S), 4.0-4.1 (m, H, NH); 13C NMR (125 MHz, CDCl3): δ = 180, 181 (C=O), 137 (Cnaphthoquinone-NH), 134.9, 134.4, 131.1, 129.5, 127.6, 123.9, 118.3 (Caromatic, CHaromatic), 145 (Caromatic-CH), 34.9 (S-CH2 allyl), 117.2 (CH2 allyl=), 133.6 (CHallyl); +ESI: m/z= 356 [M+H]+, 173 [M-(SCH2-CH=CH2)-Ph-Cl]+, C19H14ClNO2S, (M=355.84 g/mol).
Results and discussion
In this study, new N,S-substituted naphthoquinone analogues 2, 4, 6 and 8 were obtained from the substitution reaction of previously known N-substituted naphthoquinone compounds 1 (Kalmayer et al., 1991), 3 (Tadashi et al., 1996), 5 (Vanallan et al., 1963) and 7 (Fries et al., 1922) with allyl mercaptan. Due to the chlorine atom in the skeletal structure of the synthesized N-substituted naphthaquinone derivatives, the substitution reaction with allyl mercaptan was easily realized. The synthesized and characterized chemical structures of naphthoquinone analogues are presented in Scheme 1.
Synthetic pathway of novel N,S-substituted napthoquinone analogues (2, 4, 6 and 8).
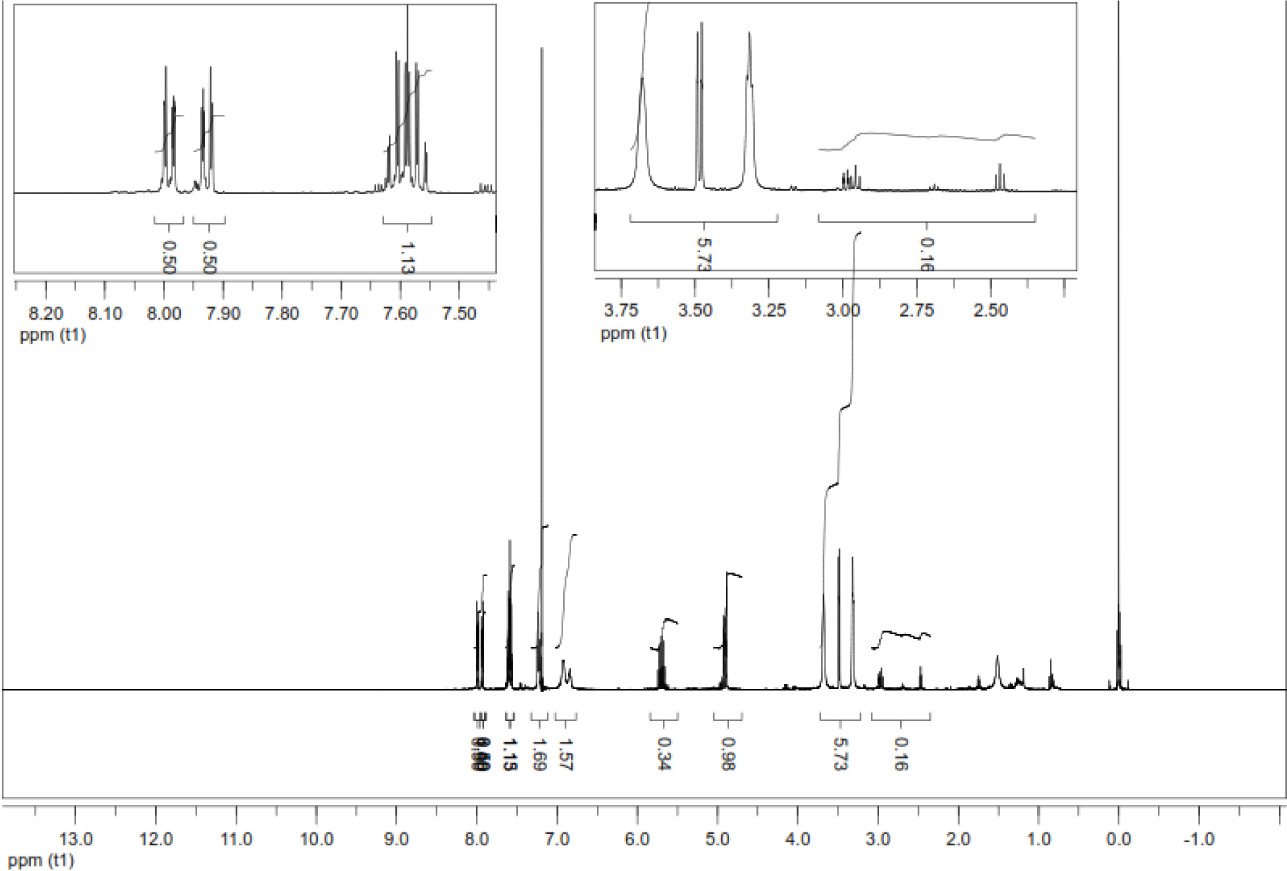
In the 1H-NMR spectrum of compound 2, the characteristic (CH=) proton of the allyl group gave a multiplet signal at δ= 5.6-5.8 ppm and (=CH2) protons gave a multiplet signal at δ= 4.9-5.1 ppm. Methylene protons in the piperazine ring were observed as multiplet at δ= 3.2-3.7 and δ= 2.4-2.9 ppm (Figure 1).
Figure 1. 1H-NMR (CDCl3) spectrum of compound 2.
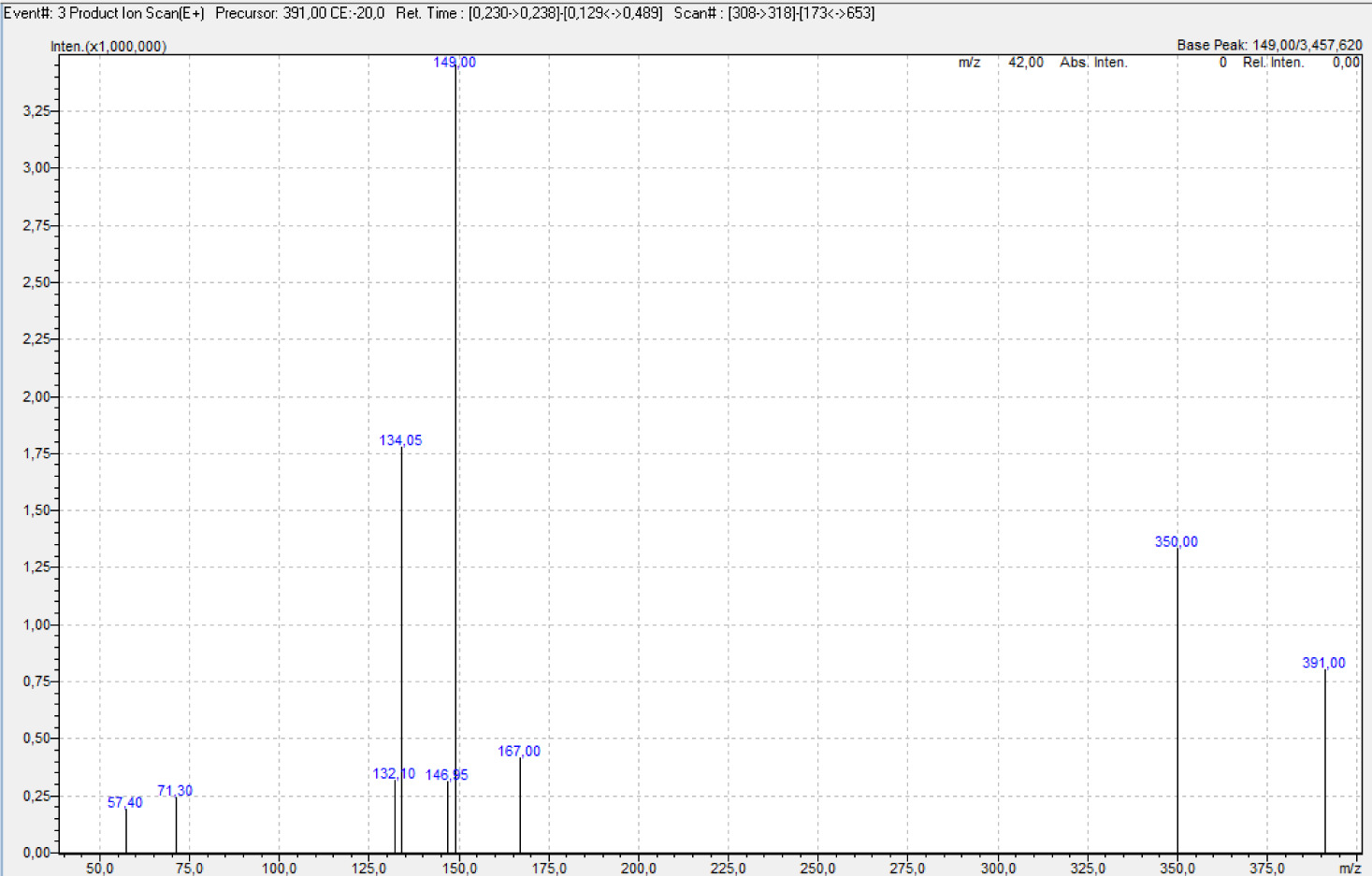
In the 13C-NMR spectrum of compound 2, the carbonyl carbons in the molecule were observed at δ= 182.1, 181 ppm, the tertiary carbon of the allyl group at δ= 133.9 ppm and the (=CH2) carbon at δ= 117 ppm. Piperazine methylene carbons were seen at δ= 52.4 ppm and the methylene carbon attached to the sulfur atom was seen at δ= 37.7 ppm. In the mass spectrum of compound 2, m/z= 391 [M+H]+ molecular ion and m/z= 350 [M-CH2-CH2-CH=CH2] molecular fragmentation ion was observed with the breakage of the allyl (CH2-CH=CH2) group from this molecule (Figure 2).
Figure 2. MS spectrum of compound 2.
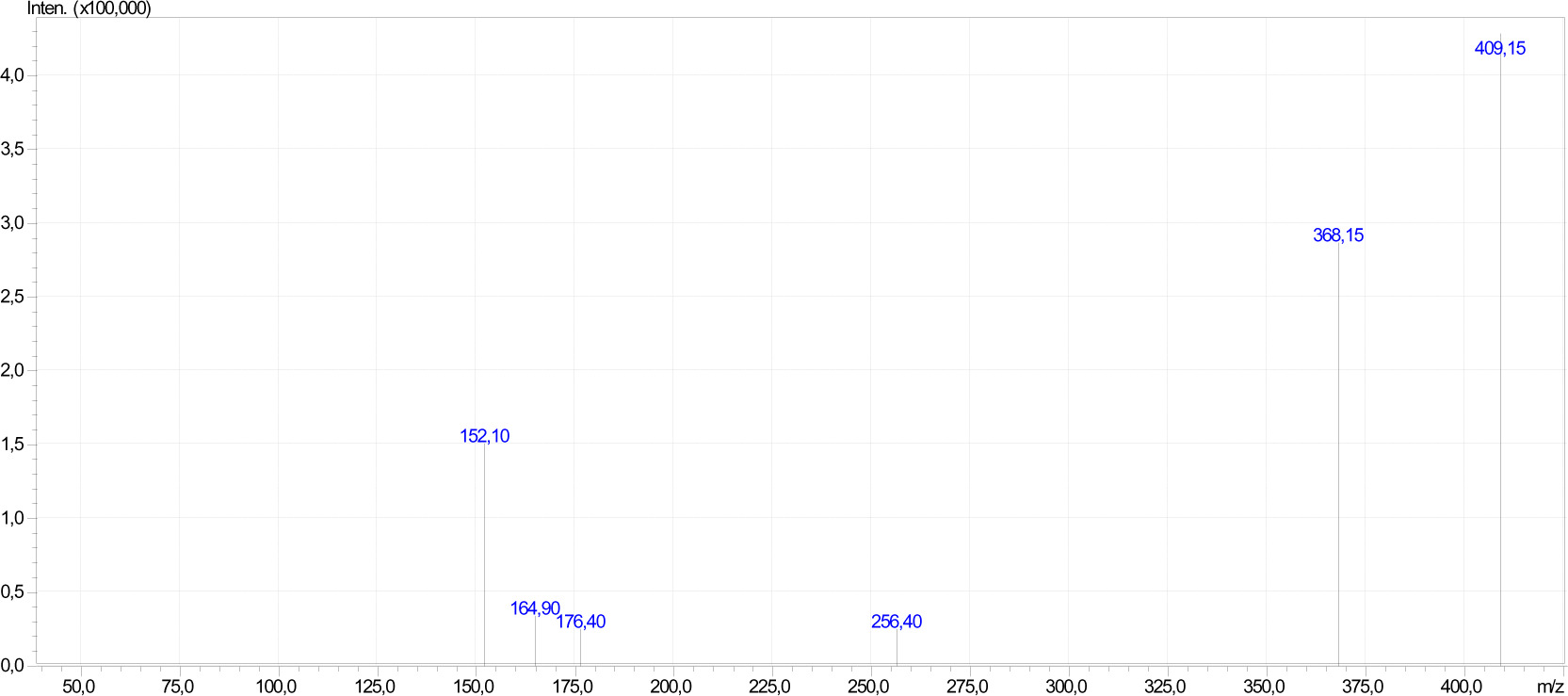
In the 1H-NMR spectrum of compound 4, the aromatic protons of the fluorophenylpiperazine group were multiplet at δ= 6.8-7.0 ppm, the characteristic (CH=) proton of the allyl group was multiplet at δ= 5.6-5.8 ppm and (=CH2) protons were multiplet at δ= 4.8-5.0 ppm (Figure 3). In the mass spectrum of compound 4, m/z= 409.15 [M+H]+ molecular ion and m/z= 368.15 [MCH2-CH2-CH=CH2]+ molecular fragmentation ion was observed with the breakage of the allyl (CH2-CH=CH2) group from this molecule (Figure 4). In the 13C-NMR spectrum of compound 4, the carbonyl carbons in the molecule were observed at δ= 182.2, 182.1 ppm, the quaternary carbon in the naphthoquinone ring attached to the sulfur atom at δ= 126.8 ppm, the quaternary carbon attached to the piperazine ring at δ= 154.9 ppm, the piperazine methylene carbons at δ= 51.7 ppm and the methylene carbon attached to the sulfur atom at δ= 37.7 ppm. In the mass spectrum of compound 6, m/z= 404 [M+H]+ molecular ion and m/z= 331 [M-SCH2-CH2-CH=CH2]+ molecular fragmentation ion was observed with the breakage of the allylthiol (SCH2-CH=CH2) group from this molecule.
Figure 3. 1H-NMR (CDCl3) spectrum of compound 4.

Figure 4 . MS spectrum of compound 4.
In the 1H-NMR spectrum of compound 6, the aromatic protons in the benzylpiperidine ring gave multiplet signals at δ=7.0-8.0 ppm, the characteristic (CH=) proton of the allyl group gave multiplet signals at δ= 5.6-5.9 ppm and (=CH2) protons gave multiplet signals at δ= 4.9-5.2 ppm (Figure 5). In the 13C-NMR spectrum of compound 6, the quaternary carbon in the naphthoquinone ring attached to the nitrogen atom in the molecule was observed at δ= 143.4 ppm, the methylene carbons in the piperidine ring at δ= 29.3 ppm and the quaternary carbon at δ= 25.4 ppm.
Figure 5. 1H-NMR (CDCl3) spectrum of compound 6.
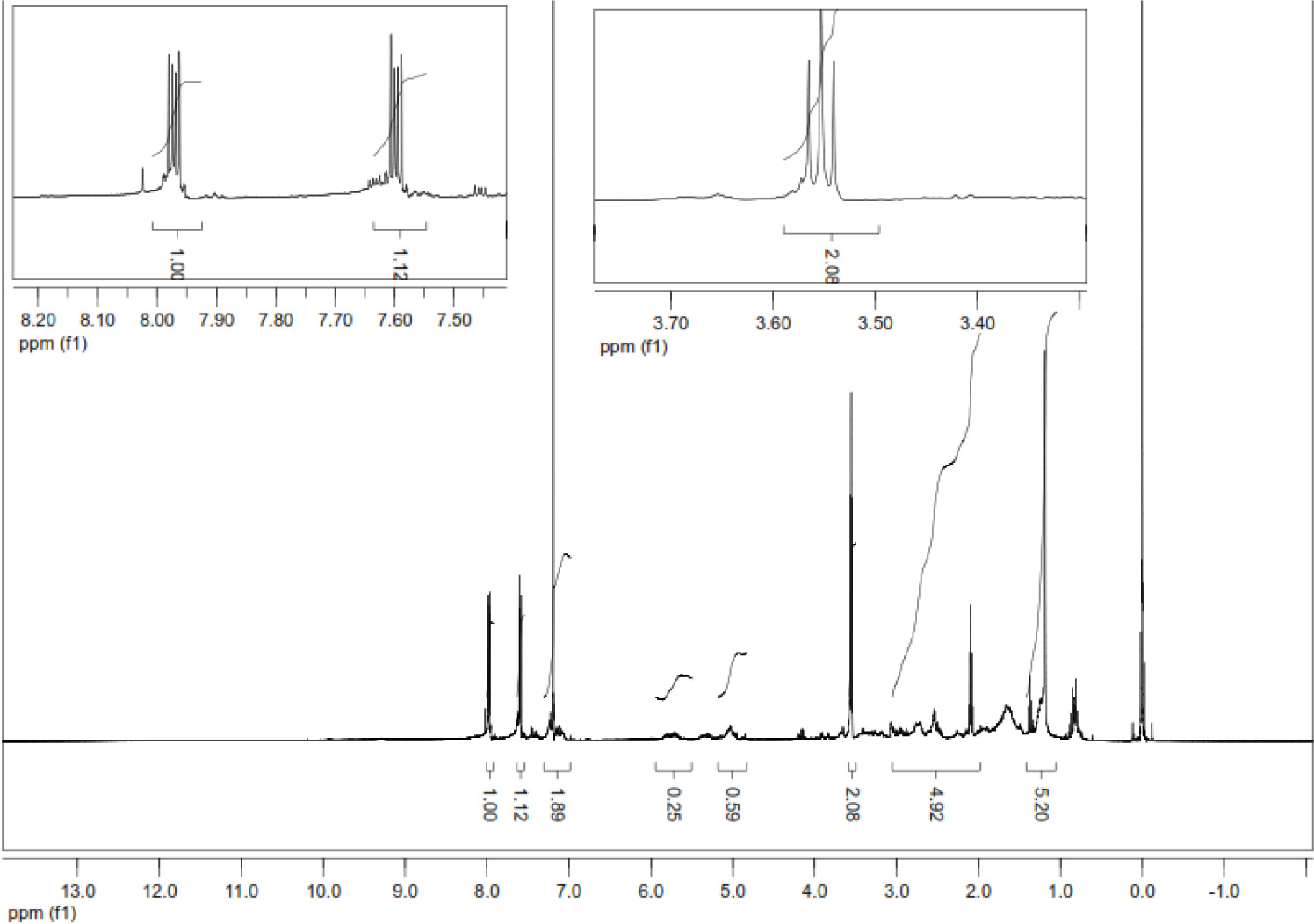
In the mass spectrum of compound 8, m/z= 356 [M+H]+ molecular ion was observed (Figure 6). In the 1H-NMR spectrum of compound 8, aromatic protons in the 4-chlorophenylamino ring were multiplet at δ=6.8-7.3 ppm, the characteristic (CH=) proton of the allyl group was multiplet at δ= 5.6-5.7 ppm, (=CH2) protons were multiplet at δ= 4.9-5.0 ppm. In the 13C-NMR spectrum of compound 8, the carbonyl carbons in the molecule were observed at δ= 180, 181 ppm, the quaternary carbon of the naphthoquinone ring attached to the nitrogen atom was observed at δ= 137 ppm and in the FT-IR spectrum of the compound, the characteristic stretching band of the NH group at υ= 3319 cm-1 was observed (Figure 7).
Figure 6. MS spectrum of compound 8.
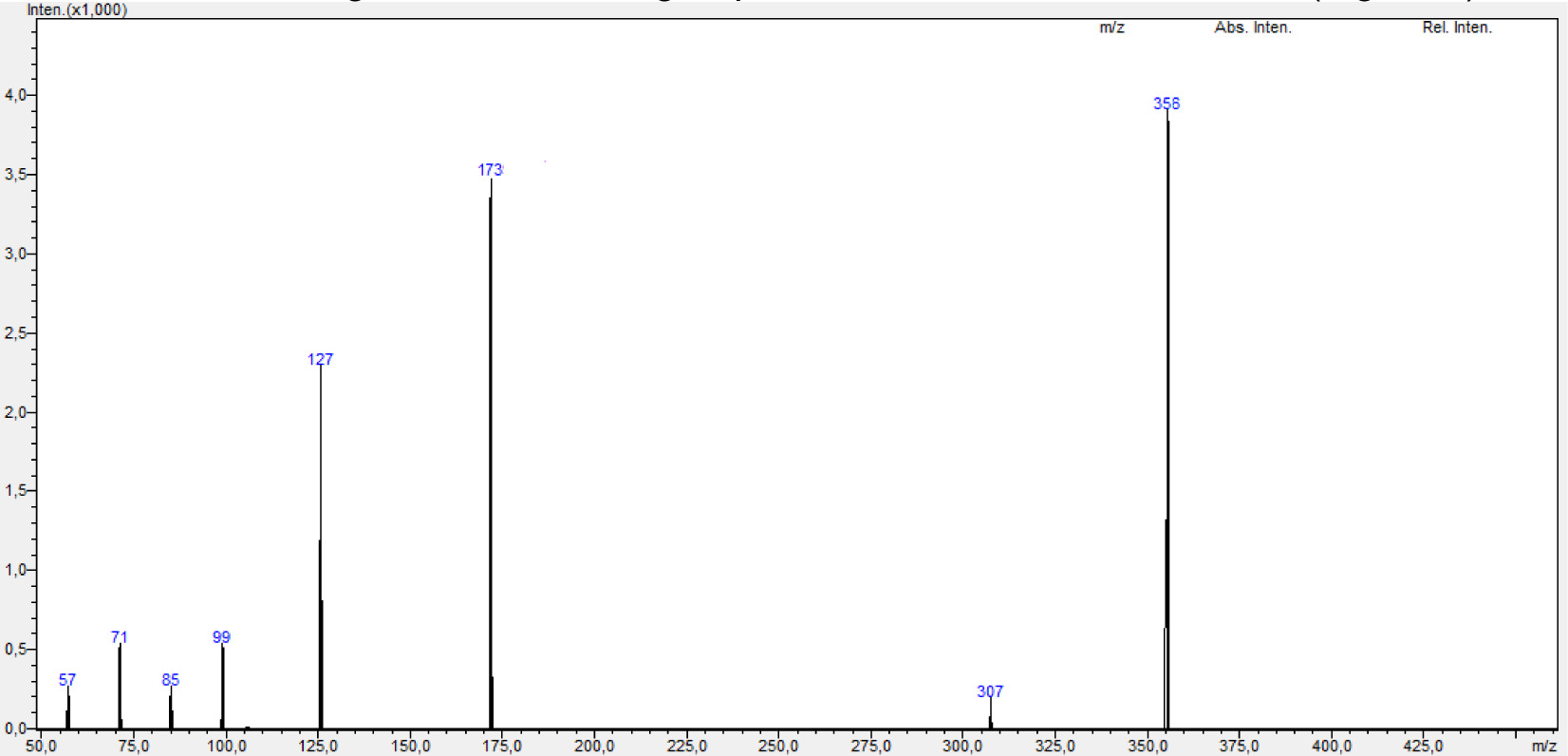
Figure 7. FT-IR spectrum of compound 8.
Quinones are used in many areas of technology, including as pigments, as drugs, in microbial fuel cells and in supramolecular chemistry. Biosynthetically, quinones can be synthesized in bioreactors and, thanks to their structural richness, they are preferred for industries that use this advantage, for example in the search for new antibiotics, cancer drugs, food colourants and textile dyes (Christiansen et al., 2021). Quinones play a key role as charge storage electrode materials due to their redox centre and integration into the material flow in the biosphere. Effective electrical conductors and quinone-based biomaterials can greatly enhance electrical energy storage systems and technologies. Quinones are used, for example, in the creation of biopolymer energy storage systems using renewable biohybrid electrodes (Ajjan et al., 2019).
Conclusions
The biological activities of natural or synthetic naphthoquinone compounds containing amino and thio groups have been known for many years. The active properties of the naphthoquinone compounds are due to their redox ability. The aim of this study is to synthesize amino- and thiosubstituted naphthoquinone molecules based on previously known aminonaphthoquinone analogues with biological activity. In the structures of the new compounds obtained, there are groups such as amino, thioether, fluorine, chlorine with high biological activity in the literature. We expect that new N,S-substituted naphthoquinone analogues will provide a basis for pharmaceutical chemistry studies.